Optimising heart substructure visibility in 4D planning CT by compensating respiratory motion
PO-1676
Abstract
Optimising heart substructure visibility in 4D planning CT by compensating respiratory motion
Authors: Marcel van Herk1, Tom Marchant2, Ed Henderson1, Donal McSweeney3, Azadeh Abravan1, Eliana Vasquez Osorio1, Alan McWilliam1, Corinne Faivre-Finn1, Gareth Price1
1University of Manchester, Division of cancer sciences, Faculty of biology, medicine and health, Manchester, United Kingdom; 2The Christie NHS Trust, Radiotherapy, Manchester, United Kingdom; 3University of Manchester, Division of cancer sciences, Faculty of biology, medicine and health , Manchester, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Excess radiation dose to cardiac sub-structures in the base of the heart has been associated with worse survival. These studies suggest that plans that are optimised to reduce dose to these structures may reduce the risk of radiation induced cardiac toxicity. However, substructure delineation is not an easy task because the heart moves both due to respiration and heartbeat. The purpose of this study is to evaluate substructure detection in the base of the heart for different existing post-processing methods that correct for respiratory motion in routine 4D planning CTs.
Material and Methods
4D planning CTs were collected from 26 lung cancer patients treated with radiotherapy between January and March 2021. Heart substructures were segmented with an efficient in-house developed auto-contouring package trained on manually delineated structures of 42 patients on the average scan (including right atrium, proximal coronary arteries and aortic valve). We assume that the clearest images will result in the lowest failure rate for auto-contouring and lead to more consistent contours between patients (evaluated as Standard Deviation / Mean of contour volumes). We tested the following post-processing methods: 1) Use of the 0% phase; 2) Regular average 4D CT scan; 3) MIP scan; 4) Average pixel values after deforming all scans in the 4D CT to mid-position; 5) Maximum pixel values after mid-position; 6) Median pixel values after mid-position deformation. Deformable registration for mid-position calculation was performed using a published phase-based optical flow method. Segmentation was classified as failure if the heart sub-structures were not detected automatically.
Results
The improvement of heart substructures by motion compensation for respiratory motion on visual observation was limited and relates to respiration-driven heart motion, which at the base of the heart varied between 1 and 10 mm. An example for a patient with 8 mm peak-peak motion is shown in Fig. 1. Post-processing made the largest difference to visibility of the coronary arteries. Most segmentation failures occurred for the right coronary artery, where the mid-position median method had 8% failures, followed by mid-position average and 0% phase both with 12% failures, while the average scan had 16% failures (Fig. 2). Both methods based on maximum intensity had 40% failures. The most consistent contours were produced by the mid-position median, mid-position average and regular average methods (Fig. 2).
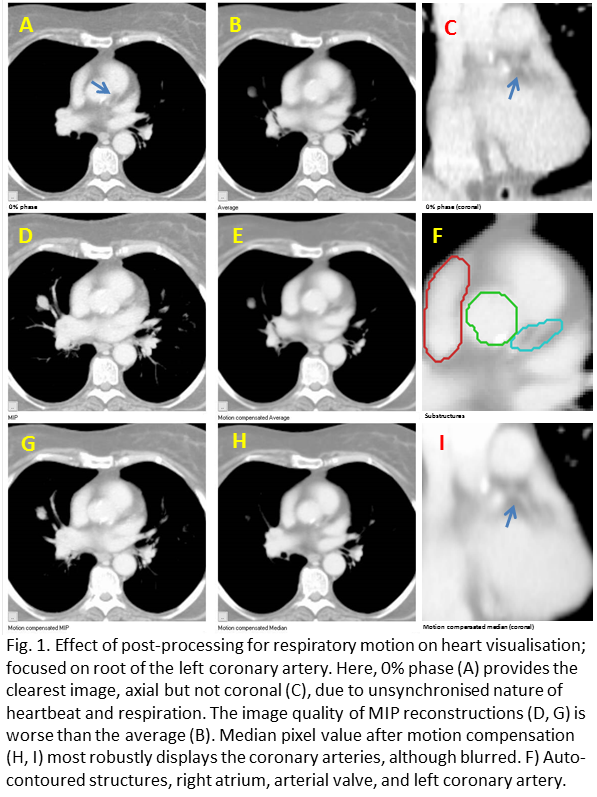
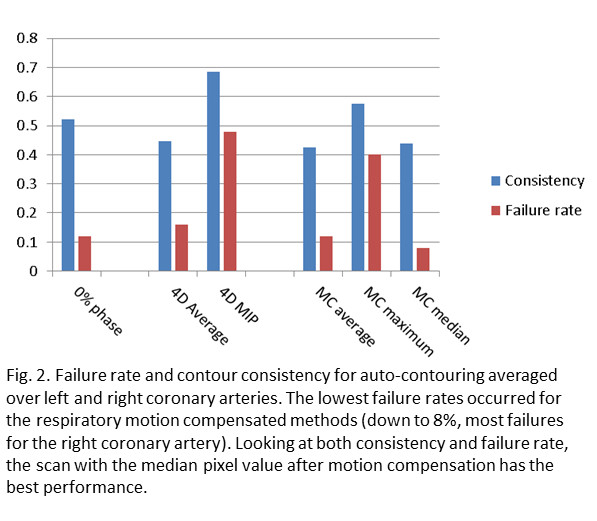
Conclusion
Compensation of respiratory motion improves segmentation of heart substructures, with the best performance for the mid-position median method. The advantage of taking the median pixel value is likely that it will show the most stable heart position i.e. at diastole. For further improvements cardiac motion has to be addressed and the model should be trained on motion compensated data. These are important steps towards the routine implementation of a dose limit for sub-structures situated in the base of the heart.