Temozolomide followed by chemoradiotherapy versus upfront chemoradiotherapy in Glioblastoma
Azadeh Sharifian ,
Iran Islamic Republic of
PO-1124
Abstract
Temozolomide followed by chemoradiotherapy versus upfront chemoradiotherapy in Glioblastoma
Authors: Reza Ghalehtaki1, Azadeh Sharifian1, Ahmad Pour-Rashidi2, Ghazaleh Amjad3
1Tehran University of Medical Sciences, Radiation Oncology, Tehran, Iran Islamic Republic of; 2Tehran University of Medical Sciences, Neurosurgery, Tehran, Iran Islamic Republic of; 3Iran University of Medical Sciences, Radiology, Tehran, Iran Islamic Republic of
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiation therapy (RT) with concurrent and adjuvant temozolomide (TMZ) is the mainstay adjuvant treatment for Glioblastoma multiform (GBM). RT planning and preparation is a sophisticated and time-consuming process that may alter treatment outcomes, especially among those with considerable residual disease after resection. Here, we investigated the efficacy of neoadjuvant TMZ followed by standard chemoradiotherapy (CRT).
Material and Methods
Adult patients with pathologically proven GBM were recruited in our study. Patients were randomized into two groups. The first group was the control group which includes the standard upfront CRT consisting of 60Gy in 30 fractions of RT and concurrent TMZ (75mg/m2) and adjuvant TMZ (150-200 mg/m2). The second group that we call neo-TMZ so on, includes 3 courses of neoadjuvant TMZ (150-200 mg/m2) followed by standard CRT as described in the control group. In case of clinical deterioration, the chemotherapy was withheld and RT started as soon as possible. Both groups underwent adjuvant TMZ (200mg/m2) with the duration at the discretion of the physician. The primary outcome was progression-free survival (PFS) starting from the time of randomization. The trial design was approved by the institutional review board and ethics committee (IR.TUMS.IKHC.REC.1399.240). We registered our trial in IRCT repository (IRCT20150929024266N5).
Results
Due to the very slow accrual rate, we stopped the patient recruitment after accruing 35 patients (16 neo-TMZ and 19 control). The median performance status score was 1 (IQR: 1-2) and the median age was 56 (IQR: 46-65). The median follow-up period was 13 months. Eleven patients (68.8%) completed 3 courses of neoadjuvant TMZ as planned. Two patients (12.5%) received two cycles and three patients (18.8%) received only one cycle due to the progression of the tumor. The median progression-free survival (PFS) were 9 months (95% confidence interval, 4.2-13.8 months) and 3 months (95% confidence interval, 1.9-4), in the control and neo-TMZ groups, respectively (HR=0.6, [CI95%= 0.3-1.36]). The 12-month PFS rates were 25% and 17% in the control and the neo-TMZ groups, respectively. Among the MGMT methylated group, the median PFS was 3 months (95%CI, 1.9-4 months) and 3 months (95%CI=0.6-5.35), in the control and neo-TMZ groups, respectively (HR=1.6, [CI95%= 0.5-5.8]). The 12-month PFS was 20% and 38% in the control the neo-TMZ groups, respectively.
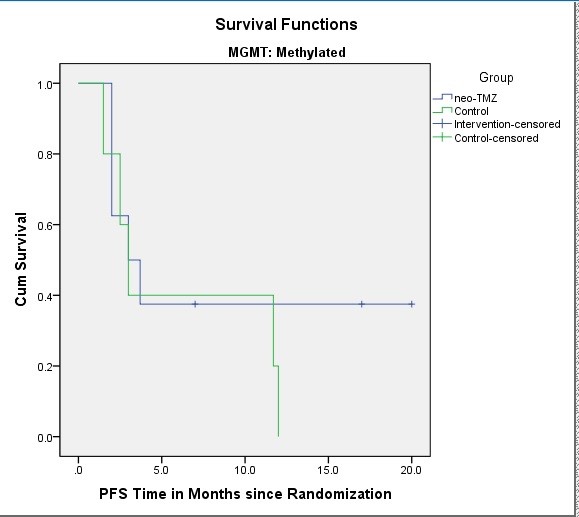
There was no grade 5 toxicity in the neo-TMZ group but there was one grade 4 and two grade 3 thrombocytopenia and neutropenia (bicytopenia). One patient had grade 5 cytopenia in the control group. One patient had pulmonary thromboembolism and another patient had grade 3 thrombocytopenia in the control group as well.
Conclusion
Based on our study there was not any benefit observed with the addition of neoadjuvant TMZ to the standard CRT. However, due to near doubled 12-month PFS rate we may say it seems that a small subset of MGMT methylated patients may benefit from neoadjuvant therapy.