Robotic stereotactic radiotherapy for ≥10 brain metastases treated with single treatment planning.
PO-1111
Abstract
Robotic stereotactic radiotherapy for ≥10 brain metastases treated with single treatment planning.
Authors: Miriam Torrisi1,2, Chiara Chissotti1,2, Chiara Lucrezia Deantoni1, Andrei Fodor1, Federica Ferrario1,2, Laura Giannini1,2, Martina Midulla1,2, Sara Broggi3, Roberta Tummineri1, Italo Dell'Oca1, Roberta Castriconi3, Flavia Zerbetto1, Claudio Fiorino3, Stefano Arcangeli4,2, Nadia Gisella Di Muzio1,5
1IRCCS San Raffaele Research Institute, Department of Radiation Oncology, Milan, Italy; 2Università degli studi Milano Bicocca, School of Radiation Oncology, Milan, Italy; 3IRCCS San Raffaele Research Institute, Medical Physics, Milan, Italy; 4ASST Monza, Department of Radiation Oncology, Monza, Italy; 5Università Vita Salute San Raffaele, School of Medicine and Surgery, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (SRT) are effective treatments for patients with multiple brain metastases. Here we report our experience with single treatment planning SRS/SRT delivered with CyberKnife® (Accuray, Sunnyvale, CA) in pts with more than 10 brain metastases.
Material and Methods
From 06/2018 to 08/2022 a total of 524 brain metastases in 24 pts (four of them treated twice) were treated with a single treatment plan SRS (10 pts) or SRT (6 pts with 3 fractions and 12 pts with 5 fractions) in our institution. Patient’s median age was 64 (41-85) years. Primary tumor was lung in 10 pts, breast in 7 pts, melanoma in 4 pts, parotids in one patient and one patients had lung and breast cancer simultaneously. Seven pts were previously treated with Whole Brain Radiotherapy (WBRT). Gross tumor volume (GTV) and organs at risk (OAR) were defined on a contrast-enhanced T1-weighted MRI fused to simulation-computed tomography (CT). A 1 mm margin was added to GTV to define Planning target volume (PTV). For 27 out of 28 treatments, steroid therapy was administrated as precautional therapy. Post-treatment MRI scans were used to assess local control (LC) and disease progression (PD) according to the Response Evaluation Criteria in Solid Tumors (RECIST) scale. Acute toxicity was assessed according to the CTCAE v5.0. Survival curves were calculated from the date of treatment by using the Kaplan-Meier (KM) method.
Results
Median number of brain metastases/patient was 15 (10-46). Median GTV 4.78 (0.46-22.33) cc, and median PTV 10.01 (1.57-32.99) cc. Median prescribed dose was 30 (24-37.5) Gy, at the 77 (66-80) % median isodose, in a median of 3 (1-5) fractions, delivered in consecutive days. With a median follow up of 4.13 (0.5-27.04) months, 19 pts were found dead. Acute toxicity was as follows: 3 pts presented G1 dizziness and one patient G2 dizziness, 2 pts G1 headache, 1 patient G1 nausea, 1 patient G1 hemiparesis (weakness), and 1 patient G1 dysgeusia. No patient presented radionecrosis. Overall Survival (OS) at 6-, and 12-months was 40.9%, and 15.3%, respectively. Six- and 12-month Local Relapse Free Survival (LRFS) was 90.4%, and 65.9%, respectively (see Fig.1). Intracranial Relapse Free Survival (IRFS) at six and 12-months were 50.7%, and 25.3%, respectively (See Fig.2).
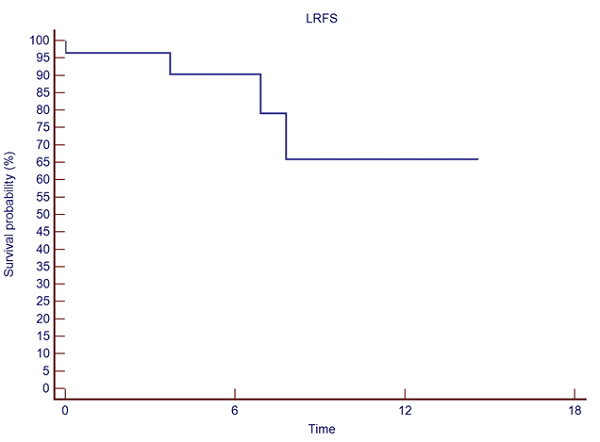
Figure 1. Local Relapse Free Survival.
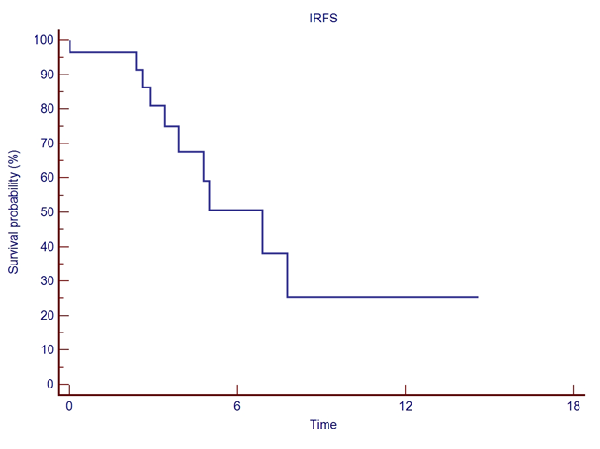
Figure 2. Intracranial Relapse Free Survival.
Conclusion
SRS/SRT can be considered a feasible treatment strategy with a good toxicity profile and excellent local control rates in pts with more than 10 brain metastases. Based on our results, careful patient selection is required.