Exploration of serum citrulline as a predictive biomarker for late bowel toxicity in prostate SABR.
Orla Houlihan,
United Kingdom
PO-1447
Abstract
Exploration of serum citrulline as a predictive biomarker for late bowel toxicity in prostate SABR.
Authors: Orla Houlihan1,2, Kelly Redmond2, Ciaran Fairmichael1,3, Ciara Lyons1,3, Conor McGarry4, Darren Mitchell1, Aidan Cole1,3, Denise Irvine4, Wendy Hyland4, Michael Hanna5, Kevin Prise3, Alan Hounsell4, Joe O'Sullivan1,3, Suneil Jain1,3
1Northern Ireland Cancer Centre, Belfast Health and Social Care Trust, Clinical Oncology, Belfast, United Kingdom; 2Queen's University Belfast, Patrick G. Johnston Centre for Cancer Research, Belfast, United Kingdom; 3Queen’s University Belfast, Patrick G. Johnston Centre for Cancer Research, Belfast, United Kingdom; 4Northern Ireland Cancer Centre, Belfast Health and Social Care Trust, Radiotherapy Medical Physics, Belfast, United Kingdom; 5Belfast City Hospital, Northern Ireland Cancer Trials Network, Belfast, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Emerging evidence suggests citrulline levels decrease during pelvic RT in response to bowel toxicity. This study explored the potential for citrulline as a predictive biomarker of late bowel toxicity in a randomised trial of SABR ± elective nodal irradiation in high risk prostate cancer (SPORT trial).
Material and Methods
30 men were randomised 1:1 to SABR to prostate-only (P-SABR) or to prostate plus pelvic lymph nodes (PPN-SABR). P-SABR patients received 36.25Gy/5 fractions/29 days. PPN-SABR patients also received 25Gy/5 fractions to pelvic nodes with the final cohort receiving a boost to the dominant intraprostatic lesion of 45-50Gy. Patient-reported EPIC summary scores and serum citrulline levels prior to first (#1), second (#2) and third (#3) fractions were quantified. A minimally clinically important change (MCIC) in EPIC score was defined as a decrease of 5 points for GI toxicity. Time to MCIC was stratified by median citrulline levels taken at #2 relative to levels at #1 and by treatment group.
Results
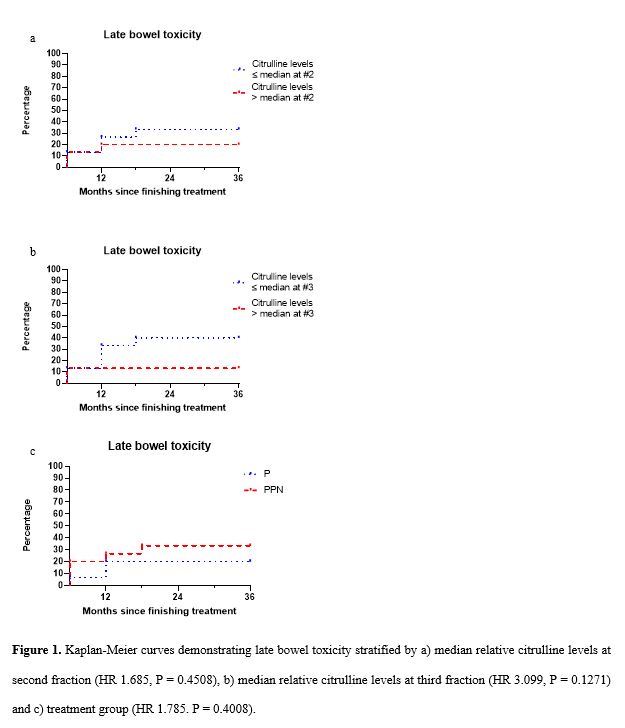
Median citrulline levels at #2 and #3 relative to #1 were 0.92 and 0.86 respectively. At 36 months, 33% of patients with relative citrulline levels ≤ median at #2 and 40% with relative citrulline levels ≤ median at #3 had experienced late bowel toxicity versus 20% with relative citrulline levels > median at #2 and 13% with relative citrulline levels > median at #3 (HR 1.685, P = 0.4508 and HR 3.099, P = 0.1271 respectively) (Fig. 1a,1b) When stratified by treatment group, 33% of patients in the PPN-SABR group had experienced late bowel toxicity versus 20% in the P-SABR group (HR 1.785. P = 0.4008) (Fig. 1c).
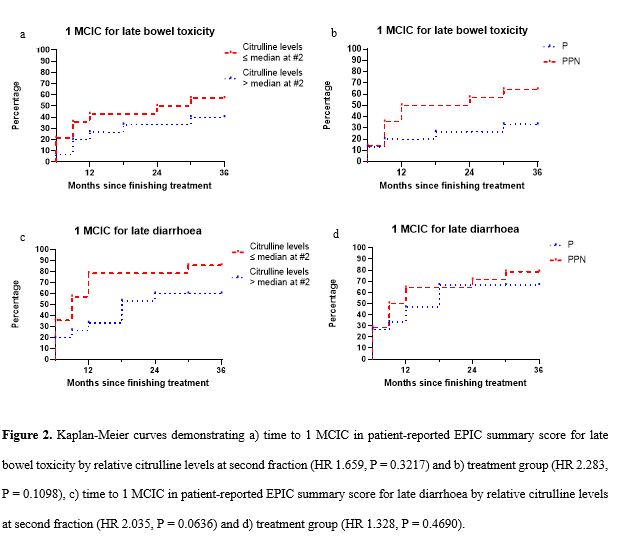
At 36 months, 57% of patients with relative citrulline levels ≤ median had experienced 1 MCIC for late bowel toxicity versus 40% of patients with relative citrulline levels > median (HR 1.659, P = 0.3217) (Fig. 2a). 64% of patients in the PPN-SABR group had experienced 1 MCIC for late bowel toxicity compared to 33% in the P-SABR group (HR 2.283, P = 0.1098) (Fig. 2b). 86% of patients with relative citrulline levels ≤ median had experienced 1 MCIC for late diarrhoea compared to 60% of patients with relative citrulline levels > median (HR 2.035, P = 0.0636) (Fig. 2c). 79% of patients in the PPN-SABR group had experienced 1 MCIC for late bowel toxicity compared to 67% in the P-SABR group (HR 1.328, P = 0.4690) (Fig. 2d).
Conclusion
More patients with relative citrulline levels ≤ median experienced late bowel toxicity and late diarrhoea than those with relative citrulline levels > median. The percentage difference in patients with 1 MCIC for late diarrhoea stratified by relative citrulline levels was greater than by treatment group. Citrulline has potential as a predictive biomarker of late bowel toxicity, particularly diarrhoea, in patients treated with prostate radiotherapy. Findings are limited by low study numbers, however, if supported by further research, citrulline levels would allow for adjustment of radiotherapy to reduce the risk of late toxicity in selected patients.