Systematic Review of Prognostic and Predictive Biomarkers in HNSCC Treated with Radiotherapy
Daniel Schanne,
Switzerland
PO-1446
Abstract
Systematic Review of Prognostic and Predictive Biomarkers in HNSCC Treated with Radiotherapy
Authors: Daniel Schanne1, Alexander Koch1, Olgun Elicin1, Roland Giger2, Michaela Medová3, Yitzhak Zimmer3, Daniel M Aebersold1
1Inselspital, Bern University Hospital and University of Bern, Department of Radiation Oncology, Bern, Switzerland; 2Inselspital, Bern University Hospital and University of Bern, Department of Otorhinolaryngology, Head and Neck Surgery, Bern, Switzerland; 3University of Bern, Department for BioMedical Research, Bern, Switzerland
Show Affiliations
Hide Affiliations
Purpose or Objective
Recent years have seen the advent of increasing numbers of biomarkers used in the treatment of oncological diseases. However, head-and-neck squamous cell cancer (HNSCC) remains a tumor entity characterized by a paucity of personalized treatments. We performed a systematic literature review of biomarkers in HNSCC patients who received radiotherapy (RT) as part of their treatment with predominantly curative intent.
Material and Methods
We searched Pubmed and EMBASE for available literature on biomarkers in HNSCC. Inclusion criteria were: 1) HNSCC, 2) ≥ 10 patients, 3) association of biomarker with relevant oncological endpoint provided, 4a) all patients treated with radiotherapy, or 4b) separate outcome reported for patients treated with radiotherapy, 5) publication date between 01 Jan 2010 and 01 Mar 2022. Exclusion criteria were: 1) Biomarker HPV/EBV- DNA, 2) HPV- status as biomarker (p16, HPV- DNA etc.), 3) studies solely based on publicly available databases (TCGA, NCDB), 4) reviews. Z curves were generated using R package zcurve to addresss publication bias. OncoKB was used for identification of potentially druggable targets.
Results
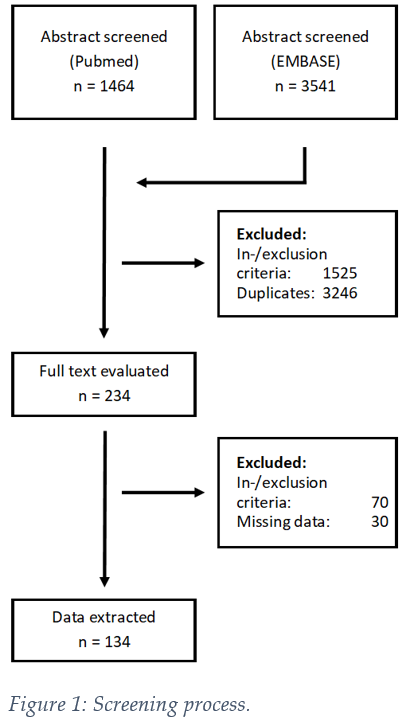
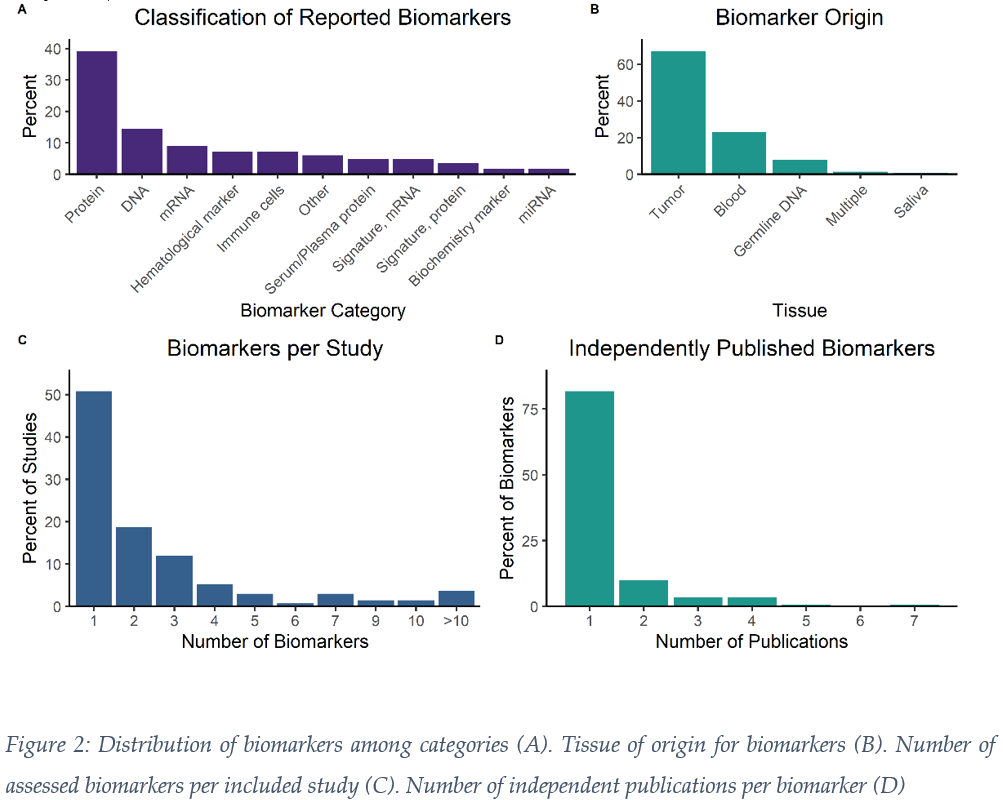
We screened 5005 publications from two databases and excluded 4771 based on inclusion criteria (Fig 1). Based on evaluation of the full-text, 134 manuscripts remained for data extraction. The most common primary tumor site was oropharynx (46%), followed by larynx (18%), oral cavity (17%) and hypopharynx (11%). 12% were early AJCC/UICC stage (I-II), whereas 81% were advanced stage (III-IV) and information was missing for 7%. Median minimum applied equivalent dose 2 Gy (EQD2) was 66 (14 – 72) Gy. The most common biomarkers were proteins (39%), DNA (14%) and mRNA (9%) (Fig 2A). 67% of biomarkers were determined in tumor tissue, 23% in blood, followed by germline DNA, saliva and others (Fig 2B). The majority of publications (51%) investigated one biomarker with a marked fall-off at two (19%), three (12%) and four or more (18%) (Fig 2C). Within our dataset, 82% of biomarkers had only been assessed in one publication (Fig 2D). Only 10% were described in two, and 8% in three or more studies. Limiting analysis to prospective data and statistically- significant results, we found three potentially druggable targets via OncoKB (therapeutic level 1-3): ERCC2, PTCH1 and EGFR. Data quality was limited: AJCC/UICC version was not mentioned in 61% of studies and AJCC/UICC stage was missing in 32%. For 34% of extracted biomarkers, only univariable analysis of outcome was available. 73% of publications were retrospective studies, 7% were from prospective randomized trials and 10% from prospective, non-randomized interventional studies. Z curves indicated the presence of publication bias.
Conclusion
There is an abundance of potential biomarkers described in HNSCC, however, data quality is limited due to retrospective data collection, univariable testing, lack of validation and publication bias. Potentially druggable biomarkers are available and should be further explored.