Understanding cGAS activation: the key to unravelling the full potential of ablative therapies?
Vera Mekers,
The Netherlands
PO-2241
Abstract
Understanding cGAS activation: the key to unravelling the full potential of ablative therapies?
Authors: Vera Mekers1, Maaike Looman1, Lune van den Boogaard1, Jan Bussink1, Marleen Ansems1, Gosse Adema1
1Radboudumc, Radiotherapy & Oncoimmunology laboratory, Department of Radiation Oncology, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy (RT) is part of standard care anti-tumor treatment in the clinic. The cGAS (cyclic GMP-AMP synthase)/STING (stimulator of IFN genes) pathway is key for the recognition of cytoplasmic DNA, released upon RT, and for the induction of immune responses following RT. cGAS was first described as a cytosolic DNA sensor, however, an increasing amount of literature describes a variety of cGAS localization patterns (membrane bound, nuclear or specific foci including localization in micronuclei or droplets), which are cell-type and condition dependent. To this date, the role of cGAS localization and activation following distinct RT schedules and how this relates to immune activation remains largely unclear. Here, we aim to study the cGAS/STING pathway in tumor cells and its effect on immune cells after distinct tumor ablative regimens.
Material and Methods
We established murine tumor cell clones (mouse colon tumor line MC38) using CRISPR-cas9 technology stably expressing cGAS fluorescent protein from its endogenous chromosomal location. These clones were subjected to thorough validation, assessing cGAS integration in the genome yielding at least 45 positive clones, followed by analysis of cGAS (fluorescent protein) expression on mRNA and protein level. Using these cells, we have now explored the effect of different RT schedules (ranging from 2 Gy to 20 Gy, single dose or fractionated) on cGAS activation and localization patterns using elaborate microscopic analysis in vitro & in vivo.
Results
We successfully developed MC38 tumor cell clones expressing endogenous levels of a cGAS fluorescent protein. In addition, the cGAS fluorescent fusion protein was fully functional as indicated by similar 1) cGAMP production 2) STING phosphorylation and 3) ISG expression upon RT as compared to wild-type cGAS. Upon RT treatment, cGAS localization strongly shifted towards micronuclear localization in vitro (figure 1) as well as following RT in vivo, resulting in a 4-fold increase (p<0.01) (figure 2) and are linked to cGAS activation. Moreover, RT induced cGAS re-localization was associated with the recruitment of immune cells into the tumor microenvironment. cGAS re-localization in tumors following different RT treatments are now quantified and related to gene expression changes in purified tumor infiltrating immune cells isolated from these same tumors.
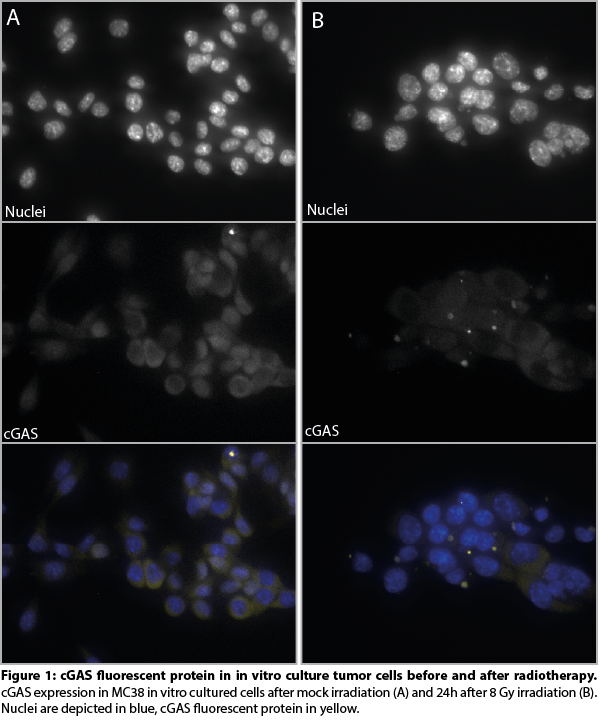
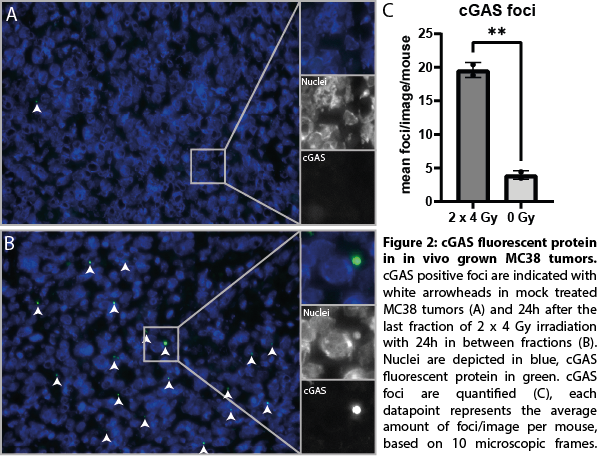
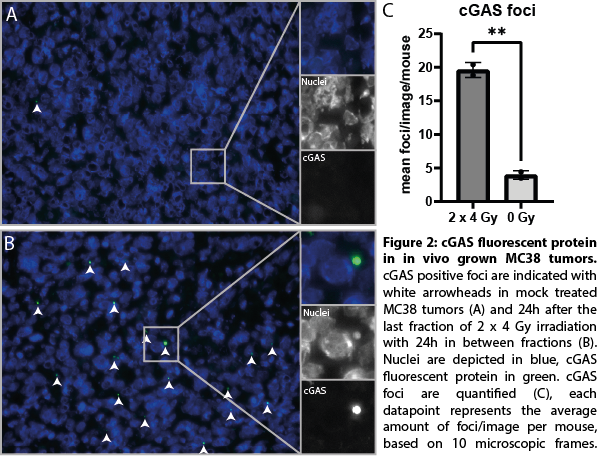
Conclusion
The tumor model expressing endogenous levels of cGAS fluorescent protein provides an unbiased tool in investigating the role of cGAS localization in cGAS/STING activation in the tumor microenvironment. These studies will yield new data on the early events following the level of cGAS re-localization and immune cell recruitment and activation and help to understand the impact of different RT treatments schedules on the immune system.