clinical outcomes of brachytherapy combined with applicator-guided IMRT in bulky cervical tumor
PO-2130
Abstract
clinical outcomes of brachytherapy combined with applicator-guided IMRT in bulky cervical tumor
Authors: Shunbin Wang1, Jinyi Lang2, Lu Shun3
1University of Electronic Science and Technology of China, School of Medicine, Chengdu, China; 2Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Department of radiation oncology, Chengdu, China; 3Department of radiation oncology, ichuan Cancer Hospital & Institute, Sichuan Cancer Center, Chengdu, China
Show Affiliations
Hide Affiliations
Purpose or Objective
To investigate the clinical outcomes and toxicity in patients with locally advanced cervical cancer treated with supplementary applicator guided-intensity modulated radiation therapy (IMRT) based on conventional intracavitary brachytherapy.
Material and Methods
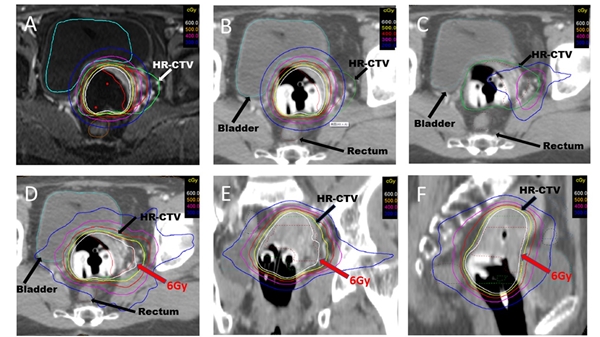
Large high risk clinical target volume (HR-CTV) volume (>40cc) at the time of brachytherapy cervical cancer patients were recruited in Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, China.. This study is a retrospective analysis of 76 patients with locally advanced cervical cancer (FIGO IIB-IVA) treated with concurrent chemo-radiotherapy followed by IC/IMRT between June 2010 and October 2016. External radiotherapy (45 Gy in 25 fractions) with cisplatin chemotherapy treated before IC/IMRT. The prescription dose for HR-CTV and IR-CTV were 6 Gy and 5 Gy per fraction for 5 fractions respectively.
Results
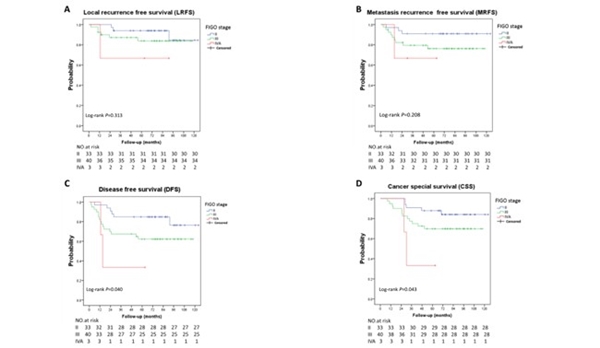
Mean HR-CTV was 65.8±23.6 cc at the time of brachytherapy. D90 for HR-CTV and IR-CTV were 88.7±3.6 Gy and 78.1±2.5 Gy. D2cc for bladder, rectum, sigmoid and small intestine were 71.8±3.8 Gy, 64.6±4.9 Gy, 63.9±5.3 Gy and 56.7±8.7 Gy respectively. Median follow-up was 85 months (47.9-124.2 months). Five-year local recurrence free survival rate, metastasis recurrence free survival rate, disease free survival rate and cancer special survival rate were 87.6%, 82.4%, 70.9% and 76.3%, respectively. The grade 1+2 gastrointestinal and urinary late toxicities were 15.8% and 21.1%, while grade 3 late toxicities were 3.9% and 5.2%, respectively. Neither acute nor late grade 4 gastrointestinal or urinary toxicities were seen.
Conclusion
The combination of ICBT with an applicator-guided supplementary IMRT boost achieved an excellent local control and overall survival with low toxicity for bulky residual cervical tumor.