Simultaneous PET and DW-MR for Outcome Prediction of Neoadjuvant Chemoradiotherapy for Rectal Cancer
PO-2072
Abstract
Simultaneous PET and DW-MR for Outcome Prediction of Neoadjuvant Chemoradiotherapy for Rectal Cancer
Authors: Jonathan Wyatt1, George Petrides2, Rachel Pearson3, Ahmed Hashmi3, Tim Simmons4, Ross Maxwell5, Hazel McCallum5
1Newcastle University, Institute of Translational and Clinical Research, Newcastle upon Tyne, United Kingdom; 2Newcastle upon Tyne Hospitals NHS Foundation Trust, Nuclear Medicine, Newcastle upon Tyne, United Kingdom; 3Newcastle upon Tyne Hospitals NHS Foundation Trust, Northern Centre for Cancer Care, Newcastle upon Tyne, United Kingdom; 4Newcastle upon Tyne Hospitals NHS Foundation Trust, Northern Centre for Cancer Care, Newcastle upon Tyne Hospitals NHS Foundation Trust, United Kingdom; 5Newcastle University, Institute for Translational and Clinical Research, Newcastle upon Tyne, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
20% of patients receiving neoadjuvant chemoradiotherapy followed by surgery for rectal cancer will have a clinical/pathological complete response. This suggests that treatment intensification strategies for the 80% of non-responders could increase the numbers of patients not requiring surgery, if they could be identified before treatment. The combination of PET and Diffusion Weighted (DW)-MR imaging has shown predictive value for treatment response which was superior to either modality on its own. This combination has been restricted to whole Gross Tumour Volume (GTV) analysis because the change in rectum position makes accurate registration between different imaging sessions challenging. Simultaneous PET-MR imaging ensures excellent image alignment, enabling tumour sub-volume analysis on PET and DW-MR parameters. This study aimed to perform a first characterisation of simultaneous PET and DW-MR for outcome prediction of neoadjuvant chemoradiotherapy for rectal cancer.
Material and Methods
7 patients with rectal cancer treated with neoadjuvant chemoradiotherapy followed by surgery received a 18F-FDG PET-MR scan in the radiotherapy position before treatment. The protocol included a simultaneous DW-MR (b-values 100, 500, 1000 s mm-2) and PET acquisition, followed by a T2-weighted image. Primary GTVs were contoured on the PET image by a consultant PET radiologist and on the T2-weighted images by consultant clinical oncologists. Whole GTV mean PET Standard Uptake Value (SUV) and DW-MR Apparent Diffusion Coefficients (ADC) were calculated. GTV sub-volumes were contoured using a threshold of 70% of SUVmax and mean SUV and ADC within this calculated. ADC maps were resampled to the PET image and voxel-wise multi-parametric scatter plots determined. Patients were divided into responders and non-responders based on post-surgery pathology.
Results
1/7 patient was a complete responder on histopathology. PET and DW-MR images showed good alignment (figure 1). Whole GTV mean SUV and ADCs were 4.5 g ml-1 and 1472 x 10-6 mm2 s-1 (responder) and 7.1 ± 1.3 g ml-1 and 1204 ± 160 x 10-6 mm2 s-1 (non-responders). Sub-volume GTV mean SUV and ADCs had larger differences: 6.1 g ml-1 and 1260 x 10-6 mm2 s-1 (responder) and 15.3 ± 5.0 g ml-1 and 889 ± 213 x 10-6 mm2 s-1 (non-responders). The multi-parametric scatter plot for the responder appeared different to those of the non-responders (see figure 2).
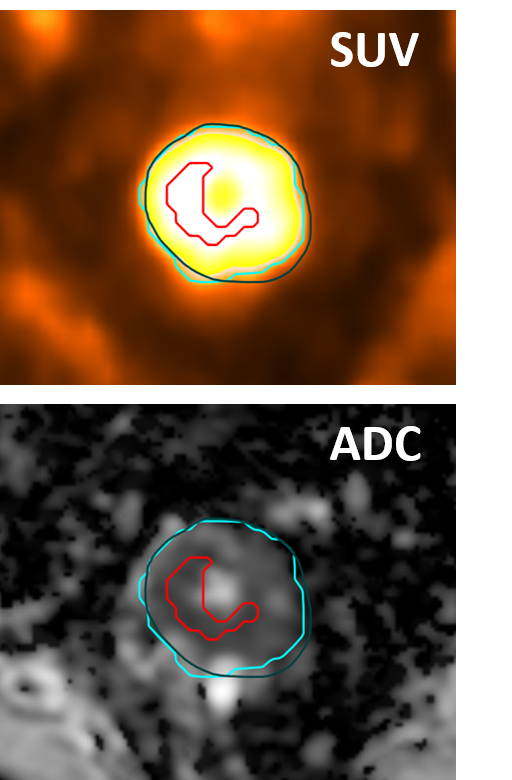
Figure 1 Example images for a non-responder, showing PET (blue), MR (green) and PET sub-volume (red) GTVs.
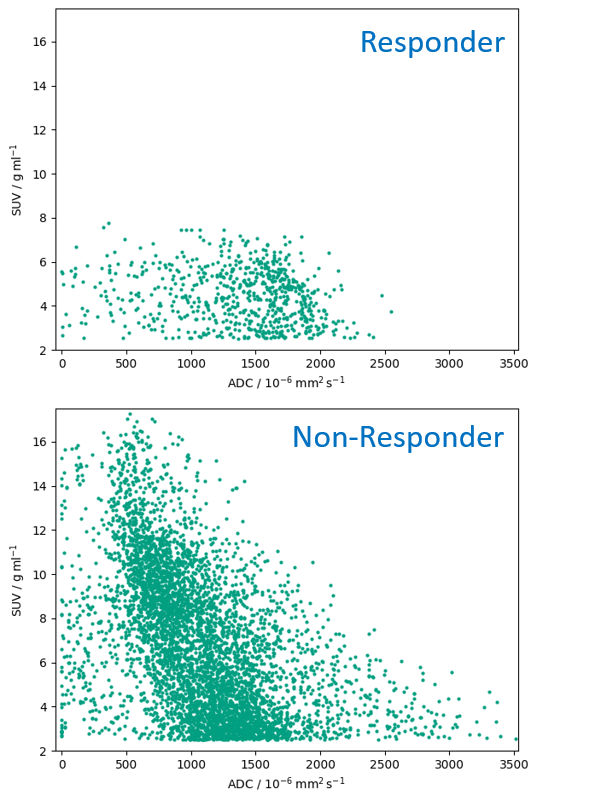
Figure 2 Multi-parametric scatter plots for the responder and a representative non-responder.
Conclusion
Simultaneous PET and DW-MR enables sub-volume analysis of rectal cancers. Potentially this will improve the predictive value for treatment response to neoadjuvant chemoradiotherapy, as well as enabling dose painting treatment strategies. Further studies are required to determine the repeatability of simultaneous PET and DW-MR imaging and evaluate the predictive value in substantially larger patient cohorts.