Radiobiological optimization and robustness evaluation for radiotherapy combined with hyperthermia
PO-1851
Abstract
Radiobiological optimization and robustness evaluation for radiotherapy combined with hyperthermia
Authors: Timoteo Daniel Herrera1, Jakob Ödén2, H. Petra Kok1, Johannes Crezee1
1Amsterdam UMC location University of Amsterdam, Radiation Oncology, Amsterdam, The Netherlands; 2RaySearch Laboratories AB, Physics, Stockholm, Sweden
Show Affiliations
Hide Affiliations
Purpose or Objective
Hyperthermia (HT) has a radiosensitizing effect which can be quantified using the LQ-model with temperature-dependent parameters. An enhanced equivalent radiation dose (EQDRT) is obtained, corresponding to the dose needed with radiotherapy (RT) alone to have the same effect. Taking HT temperature distribution as an input, the RT plan can be optimized and evaluated computing EQDRT voxelwise. The resulting dose distribution will depend on temperature levels achieved in the HT treatment and time interval between modalities. Aim of this work is to evaluate EQDRT robustness against realistic clinical HT scenarios.
Material and Methods
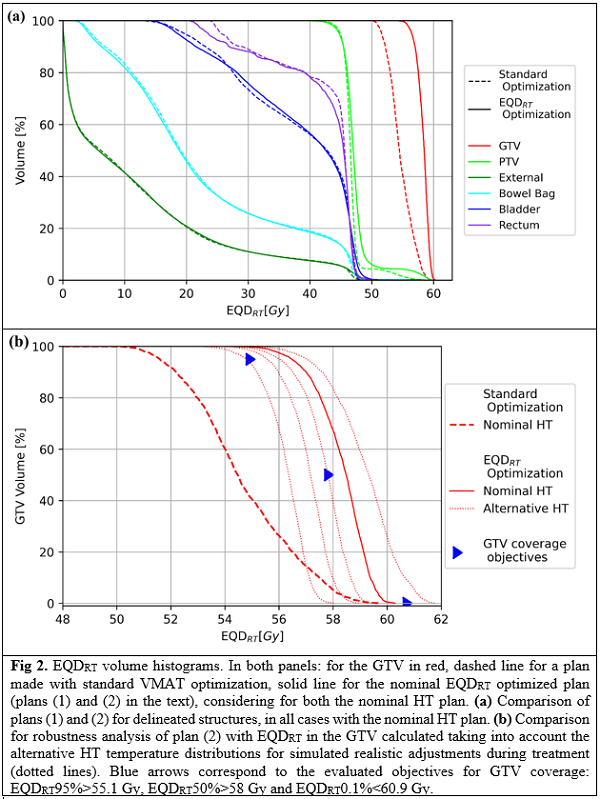
A nominal HT plan was optimized with Plan2Heat for a cervical cancer case. Four alternative HT plans were then computed to simulate realistic adjustments during treatment. Resulting temperature distributions were imported to a research version of RayStation 12A where 6 VMAT plans were optimized: (1) standard plan of 23x2 Gy to the PTV, (2) nominal EQDRT optimized plan accounting for the temperature distribution of the nominal HT plan, aiming for 58 Gy EQDRT in the GTV while maintaining PTV and OAR doses as in plan (1), (3-6) as plan (2) but considering the 4 alternative HT plans. D0.1% was evaluated in OARs. Subsequently, for each RT plan, 5 EQDRT distributions were computed, one with each HT plan. Robustness of the plans was analyzed evaluating in the GTV, for every EQDRT, the objectives: homogeneity index (HI)>0.95, EQDRT95%>55.1 Gy, EQDRT50%>58 Gy and EQDRT0.1%<60.9 Gy.
Results
Plan (2) achieved more homogeneous EDQRT in the GTV by voxelwise adjusting the RT dose driven by thermal effects, while ensuring coverage and homogeneity in the PTV (Fig. 1). EQDRT95% of the GTV increased from 51.6 Gy to 57.3 Gy, and HI from 0.89 to 0.96, with respect to plan (1) (EQDRT with nominal HT plan). In robustness analysis, for the 5 EQDRT distributions computed with plan (2), (3), (4), (5) and (6); 14, 13, 14, 13 and 11 out of 20 objectives were in tolerance, respectively. Plan (2) was preferred because of higher HI and lower EQDRT0.1% overall. Ranges for objectives in plan (2) were: EQDRT95% 54.8-57.0 Gy, EQDRT50% 56.4-59.3, EQDRT0.1% 57.9-61.6 Gy, HI 0.93-0.96 (Fig. 2). All objectives (except plan (5) HI=0.94) were met when each RT plan was combined with the HT it was optimized for. OARs’ DVH shape in plans (2-6) was similar to plan (1), while D0.1% was increased from 47.1, 47.1, and 46.9 Gy to ranges 48.6-50.0, 48.7-50.1 and 48.5-49.4 Gy for bowel bag, bladder and rectum, respectively.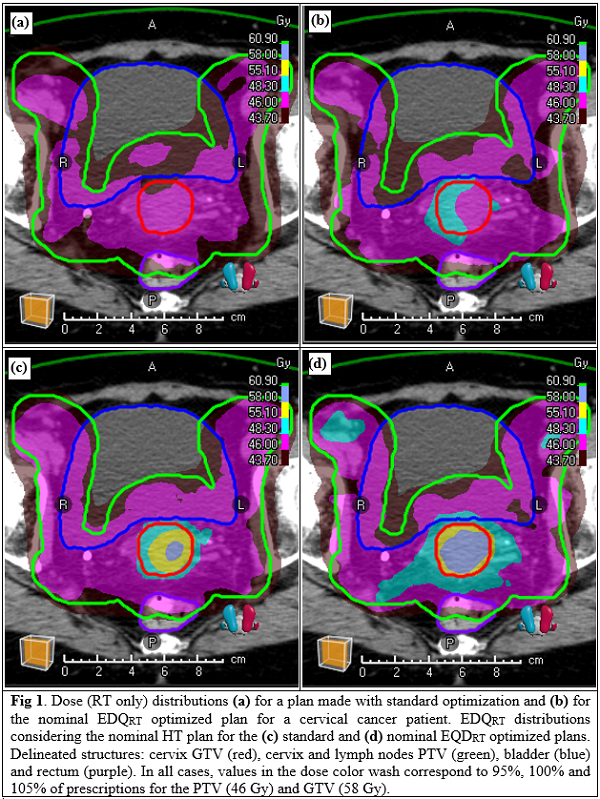
Conclusion
Accounting for thermal effects voxelwise allows to control both EQDRT level and homogeneity during RT optimization, which is not possible for standard planning. A risk of adjustments during treatment is that EQDRT objectives for GTV coverage are no longer met. This can be avoided by having alternative RT plans optimized with HT plans taking into account realistic adjustment scenarios. OARs’ DVH shape can be kept similar to standard planning, while D0.1% can increase up to 3 Gy.