Examining the FLASH effect by performing radiobiological Monte Carlo simulations with TOPAS-nBio
PO-1850
Abstract
Examining the FLASH effect by performing radiobiological Monte Carlo simulations with TOPAS-nBio
Authors: Larissa Derksen1, Veronika Flatten1,2, Rita Engenhart-Cabillic2,3, Klemens Zink1,2,3, Kilian-Simon Baumann1,2,4
1University of Applied Sciences, Institute of Medical Physics and Radiation Protection, Giessen, Germany; 2University Medical Center Giessen-Marburg, Department of Radiotherapy and Radiooncology, Marburg, Germany; 3Marburg Ion-Beam Therapy Center, MIT, Marburg, Germany; 4Marburg Ion-Beam Therapy Center , MIT, Marburg, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
In radiotherapy (RT), there is a permanent compromise to be found between the greatest tumor control and the least normal tissue toxicity. To best achieve both, new techniques are constantly under development. One promising new technique includes the so-called FLASH-RT applying ultra-high dose rates (≥ 40 Gy/s). Here, a reduced normal tissue toxicity while maintaining the same tumor control has been observed in comparison to conventional dose rates. However, this FLASH effect cannot be fully explained yet. One explanation might be that interactions between the chemical molecules produced by water radiolysis of different primary ionizing particles, so-called inter-track interactions, trigger this effect. In this work, we studied the yield of chemical molecules (G-value) and the resulting DNA damages produced by ionizing particles in dependence of the dose rate by performing radiobiological Monte Carlo simulations.
Material and Methods
TOPAS-nBio was used to examine the influence of inter-track interactions on the G-value of various chemical radicals for a simple source geometry emitting 60 eV electrons isotropically. The realization of different dose rates, which cannot be set by default in TOPAS-nBio, was achieved by a different number N of primary particles that are simulated simultaneously so that the chemicals of these different particle tracks can react with each other. A larger number of primary particles simulated simultaneously corresponds to a higher dose rate. In further simulations, we studied G-values as a function of N as well as resulting DNA damages using a proton beam of 10 and 100 MeV.
Results
Figure 1 exemplarily shows the G-values as a function of N using an isotropic electron source. Increasing the dose rate, i.e., the number of tracks simulated simultaneously, decreases the G-value of OH●, H3O and eaq whereas the G-value of H2O2, OH- and H2 increases at the end of the chemical stage for the electron and proton sources. Responsible for the change of the G-value is an increase of chemical reactions in form of inter-track interactions with increasing N. As an example, the reaction OH● + OH● → H2O2 increases significantly with N reducing the amount of OH● while increasing the yield of H2O2 molecules. As a result, less reactions of OH● at the DNA occur reducing the amount of indirect DNA damages significantly (see figure 2).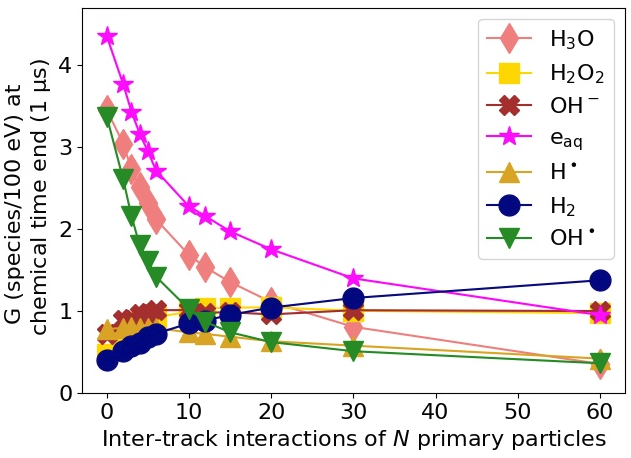
Figure 1: G-value for different chemical species at end of the chemical stage at t = 1 µs using a 60 eV electron source.
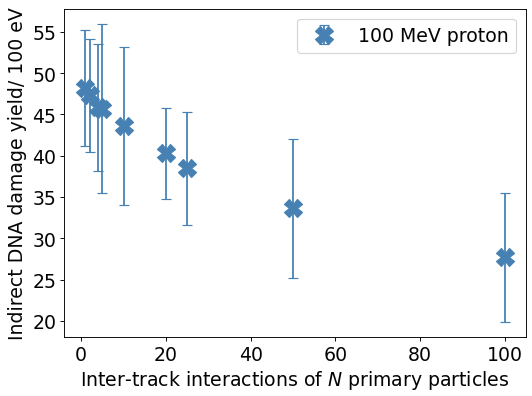
Figure 2: Total number of indirect DNA damages using a 100 MeV proton beam.
Conclusion
Inter-track interactions resulting in a variation of the yield of chemical species and DNA damages, may be a factor included in explaining the FLASH effect. To verify this hypothesis, further simulations applying our method are ongoing to investigate the DNA damage yield including dissolved oxygen and biological repair mechanism.