Personalized quantification of energy deposition during photobiomodulation treatments
PO-1842
Abstract
Personalized quantification of energy deposition during photobiomodulation treatments
Authors: Pauline MAURY1,2, Yacine Boualga1,2, Camelia Billard-Sandu3, René-Jean Bensadoun4, Eric Deutsch1,2, Charlotte ROBERT1, Charlotte Robert2
1Gustave Roussy, Radiotherapy, Villejuif, France; 2Gustave Roussy, INSERM, Radiothérapie Moléculaire et Innovation Thérapeutique, Villejuif, France; 3Gustave Roussy, Oncology-Radiotherapy, Villejuif, France; 4Centre de Haute Energie, Radiotherapy, Nice, France
Show Affiliations
Hide Affiliations
Purpose or Objective
Oral mucositis is a painful side effect of radio and/or chimio therapies, which can severely compromise the quality of life of patients and their treatment course. Extraoral photobiomodulation has demonstrated significant benefits in several randomized clinical trials such as a stimulation of tissue regeneration or reductions of inflammation and pain. However, the physical characterization of the beam properties and the rationale for treatment parameters selection are often disregarded, as well as the quantification of the energy deposition. The present work provides a proof-of-concept showing that Monte Carlo (MC) simulations are reliable prediction tools of energy deposition generated by PBM treatments. Thus, they could allow to define optimal parameters for an accurate and personalized delivery.
Material and Methods
First, we characterized the Caremin650 device (NeoMedLight) composed of two intraoral pads. A wavelength spectrum was obtained with an optical spectrophotometer (Oceanoptics OCEAN-HDX-VIS-NIR) and the source irradiance was measured with a powermeter (S170C, Thorlabs). Measurements were made for both pads, on both sides (front/back). An in vivo measurement of the transmitted light power during a clinical protocol was performed through the volunteer's cheek by placing a pad on the inner side of the cheek and a PD300-MS detector (Ophir) on the outer side. A T1-weighted axial MRI of the volunteer was acquired and segmented into subvolumes (muscle, fat, skin). On this basis, a multilayer model representative of the volunteers' facial tissues was defined. Optical transport of photons was modeled using GATE software in which optical processes (including absorption, Rayleigh and Mie scattering, refraction, reflection, and fluorescence) were considered. The simulated transmitted power was calculated and compared to that measured in vivo.
Results
The average emission wavelength was 649.82 nm ± 8.847 nm (Figure 1A). For the selected protocol, the measured irradiance was 25.8 ± 0.8 mW/cm2, in accordance with the manufacturer’s data (27.0 mW/cm2). The composition of the cheek materialized along the green line on the MRI (Figure 1B) was determined to be equivalent to 2 mm of skin, 3 mm of fat, and 7 mm of muscle. The irradiance prediction by the simulation was equal to 0.37 mW/cm2 while the in vivo measurement was 0.34 mW/cm2 (6.8% difference). Evaluation of the impact of optical and geometric parameters on the simulation results showed that the anisotropy factor (g), scattering length (Ls) or source-to-fantom distance do not influence the photon transmission, unlike the detector position.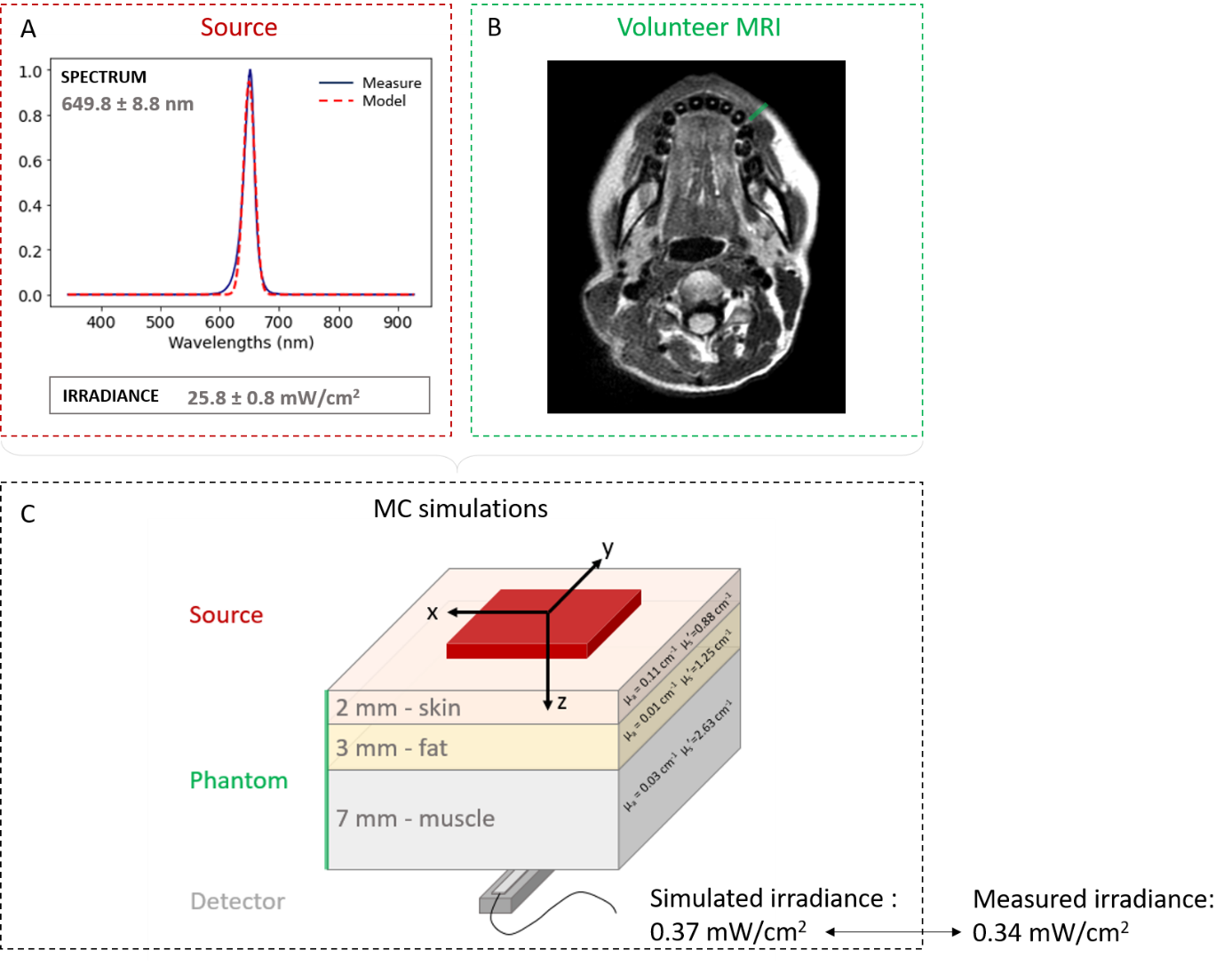
Conclusion
MC simulation is a tool to be considered for PBM treatment personalization. Currently, validation of the simulation predictions is being pursued on other volunteers with different anatomies. The next step is now to map the energies deposited by PBM treatment on a cohort of 63 head-and-neck patients to assess inter-patient variability and to establish links with reported quality of life data.