In silico identification of dose equivalences in hypofractionated prostate cancer radiotherapy
Renaud de Crevoisier,
France
PO-2095
Abstract
In silico identification of dose equivalences in hypofractionated prostate cancer radiotherapy
Authors: Carlos Sosa-Marrero1, Aurélien Briens2, Pierre Fontaine1, Oscar Acosta1, Renaud de Crevoisier1,2
1Univ Rennes, Inserm, LTSI - UMR 1099, Rennes, France; 2CLCC Eugène Marquis, Radiation Oncology, Rennes, France
Show Affiliations
Hide Affiliations
Purpose or Objective
Prostate cancer has been typically treated with a total radiation dose of 74-80 Gy administered in 2 Gy fractions during 7 or 8 weeks from Monday to Friday. In the recent years, moderate hypofractionated schedules have been proposed and tested in randomised clinical trials. Results suggest that hypofractionated radiotherapy may be recommended as a new standard for localised prostate cancer.
Nevertheless, numerous uncertainties about the dose equivalences, considering both local tumour control and toxicity, still exist. The objective of this work was thus to use a previously developed and validated in silico model of tumour response to identify dose equivalences between 2, 2.5, and 3 Gy fractionations in terms of freedom from biochemical recurrence.
Material and Methods
A cohort of 279 patients from the CLCC Eugène Marquis with localised prosate cancer and treated with EBRT was used for this study. A pre-treatment 3 T MRI scan was obtained for every individual. Sequences included T2-w. A standard hypofractionation schedule (2 Gy per fraction, with a total dose of 74-80 Gy) was administered to every patient.
In order to simulate the tumour response to radiotherapy with our in silico model, 279 2D virtual tissues representing the 279 patients of the cohort were built. Tumour volumes and average T2-w intensities were extracted from the corresponding pre-treatment MRI scans and then used to initialise the virtual tissues.
Then, the standard irradiation schedule administered to each patient of the cohort was simulated on the corresponding digital tissue. The number of virtual tumour cells at the end of treatment (8 weeks) was obtained. In previous work, it has already been shown that this simulation output is highly predictive of biochemical recurrence.
Finally, alternative 2.5 and 3 Gy fractionations (also 5 sessions per week from Monday to Friday) were explored for every patient. Virtual tissues were irradiated until they contained the same number of virtual tumour cells that after the 2 Gy schedule. Total doses needed to obtain these numbers of tumour cells were identified. The simulation was repeated 5 times on each virtual tissue and the mean output value was taken.
Results
Equivalent total doses between the three irradiation schedules are presented in Fig. 1. Median total doses of 70.6 and 64.5 Gy, were obtained for the 2.5 and 3 Gy fractionations, respectively. These results, obtained through in silico simulation, are in line with dose equivalences reported in clinical trials.
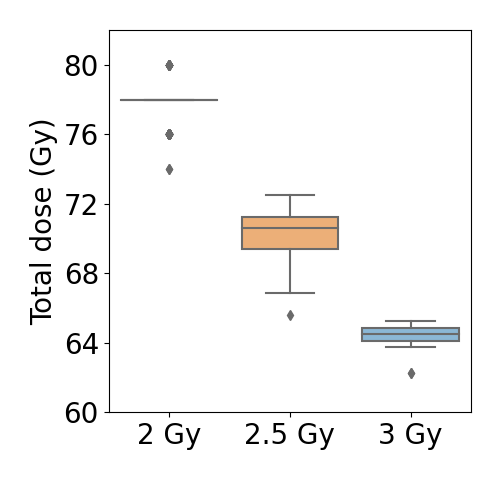
Fig 1. Equivalent total doses. Total doses for the 2 Gy fractionation correspond to those administered to the cohort. Total doses for the 2.5 and 3 Gy fractionations were obtained through in silico simulation.
Conclusion
Total dose equivalences identified by our mechanistic model of tumour response were congruent with the results reported in the clinical literature. This work paves thus the way for the identification in silico of dose equivalences between hypofractionated schedules not explored yet in clinical trials.