A radiobiological model of the synergy between radiotherapy and immunotherapy
Isabel Gonzalez-Crespo,
Spain
PO-2085
Abstract
A radiobiological model of the synergy between radiotherapy and immunotherapy
Authors: Isabel Gonzalez-Crespo1,3, Arnau Buñuel-Muriscot1, Antonio Gomez-Caamaño2, Oscar Lopez Pouso3,1, John D. Fenwick4, Juan Pardo-Montero1,5
1Health Research Institute of Santiago, Group of Medical Physics and Biomathematics, Santiago de Compostela, Spain; 2Clinical University Hospital of Santiago, Department of Radiation Oncology, Santiago de Compostela, Spain; 3University of Santiago de Compostela, Department of Applied Mathematics, Santiago de Compostela, Spain; 4Institute of Translational Medicine, University of Liverpool, Department of Molecular and Clinical Cancer Medicine, Liverpool, United Kingdom; 5Clinical University Hospital of Santiago, Department of Medical Physics, Santiago de Compostela, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
Radioimmunotherapy is a novel treatment strategy that aims at exploiting the synergy between radiotherapy and immunotherapy to increase the tumor control rates achieved with either modality alone.
The linear-quadratic (LQ) model has played a paramount role in the development of radiotherapy, assisting in the design of novel fractionations. However, because the LQ model only considers direct damage associated with radiation, it cannot be used to model response to radioimmunotherapy. There is a need to develop radiobiological models to describe the dose-effect of radioimmunotherapy treatments.
Material and Methods
We present a biomathematical model of tumor response to radioimmunotherapy, which uses the LQ/LQL models to account for direct radiation damage, and includes an immune effect. The model is written as a set of delay differential equations, with mathematical delays accounting for biological delays associated with cell death and immune cell activation and migration. The model is based on our previous work (Gonzalez-Crespo et al. 2022, DOI: 10.1109/TCBB.2022.3174454).
The model has been used to fit and analyze preclinical data (mice) of tumor response to radiotherapy combined with checkpoint inhibitors αPDL1/αCTLA4 (Deng et al. 2014, DOI: 10.1172/JCI67313; Dewan et al. 2009, DOI: 10.1158/1078-0432.CCR-09-0265).
Results
The model provides good fits to experimental volume dynamics and control data of tumors treated with radioimmunotherapy (Figure 1). Based on the results of these fits, we have investigated optimal combination schedules for radiotherapy+immunotherapy. This study suggests that the interplay between biological delays and the kinetics of the immunotherapy is important to design optimal radioimmunotherapy schedules: the combined effect seems to be lower if the immunotherapy is delivered too early or too late after radiotherapy (Figure 2).
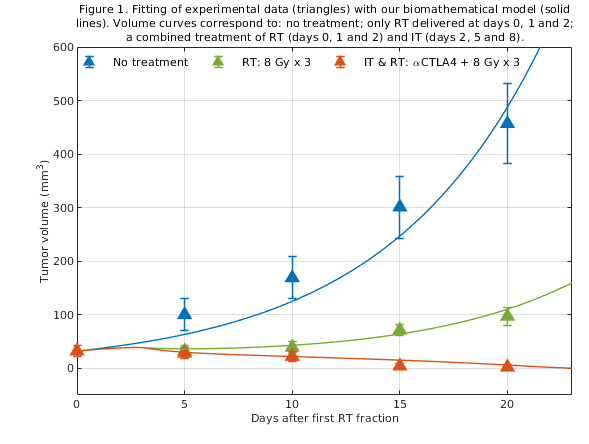
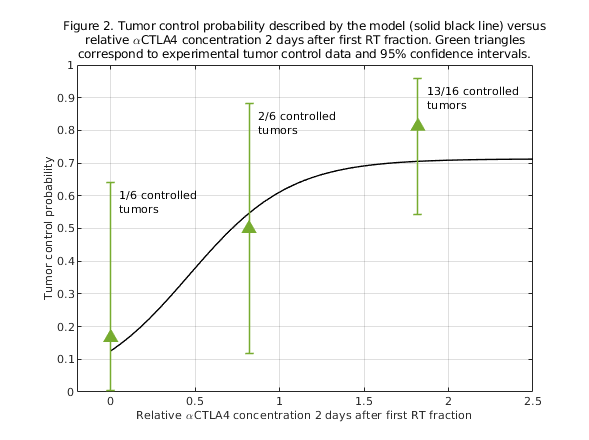
Conclusion
Developing radiobiological models of response to radioimmunotherapy is important to assist in the design of optimal administration schedules and dosages in radioimmunotherapy. Approaches like the one presented in this work constitute a stepping stone towards a better understanding of the response of tumors to radioimmunotherapy, even if these preliminary pre-clinical results must be considered with care.