Pentoxifylline and Vitamin E for radiation-induced fibrosis in breast and head and neck patients
PO-1591
Abstract
Pentoxifylline and Vitamin E for radiation-induced fibrosis in breast and head and neck patients
Authors: Martin Harpsoe1,2, Christian Nicolaj Andreassen1,2, Birgitte Vrou Offersen3,2,4
1Aarhus University Hospital, Department of Experimental Clinical Oncology, Aarhus, Denmark; 2Aarhus University Hospital, Department of Oncology, Aarhus, Denmark; 3Aarhus University Hospital, Experimental Clinical Oncology, Aarhus, Denmark; 4Aarhus University Hospital, Danish Center for Particle Therapy, Aarhus, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Patients treated with radiation therapy (RT) for breast (BC) or head and neck cancer (HNC) have an estimated 10% risk of developing fibrosis in the radiated area at 3 years. Symptoms of fibrosis may develop over years and cause pain, cosmetic deformities and decreased mobility. There is no established treatment of radiation-induced fibrosis (RIF) but pentoxyfylline and vitamin E have shown potential to alleviate RIF. Here is reported an investigation of the effect of pentoxyfylline/vitamin E on RIF in BC & HNC patients treated at a single institute.
Material and Methods
All BC & HNC patients treated and evaluated during 2016-2021 with pentoxyfylline/vitamin E for RIF at one center were identified retrospectively. Data was extracted from patient files, and all patients were handled by two consultants (BC (BV0) & BC + HNC (CNA). Therapy was oral Pentoxifylline 400mg and vitamin E 300 IE twice daily. Objective induration in the irradiated area (preferably biopsy-proven) was a prerequisite for therapy. Main endpoint was length of treatment beyond 6 months which was considered a surrogate marker of clinical benefit deemed by either the patient and/or the consultant.
Results
In total, 55 patients were referred for treatment, which was initiated in 47 patients (27 BC & 20 HNC patients). Five BC patients were lost to follow-up, whilst two HNC patients stopped treatment shortly after treatment start because of side effects, thus 40 patients were treated and evaluated. Median duration from end of radiation therapy to RIF was 30 months (BC) and 46 months (HNC). Overall, 22 BC patients were treated and 17 had clinical partial or complete response. Among 18 HNC patients, five patients had effect of the treatment. Side-effects from treatment were modest with one patient discontinuing treatment because of gastrointestinal symptoms with nausea and diarrhea and one because of headache and concentration difficulty. One BC patient who had had effect of the treatment was discontinued after approximately 9 months because of a small retina bleeding without sequelae.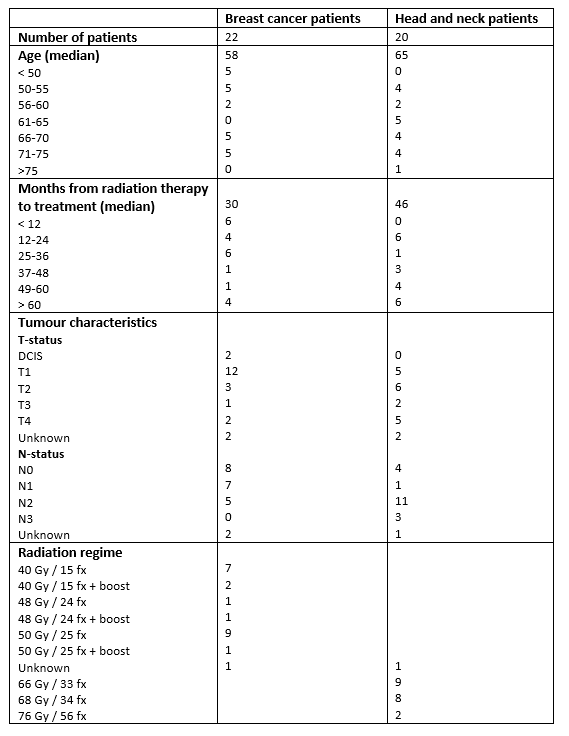
Conclusion
Pentoxifylline and vitamin E is a cheap and well-tolerated treatment and also an effective treatment in a considerable number of patients for an otherwise substantial side-effect to radiation therapy. The effect seems in our study to be more pronounced within the BC patients compared to the HNC group, but patients in both groups have benefit of the treatment. More studies are needed to investigate whether our findings also can be reproduced in larger populations and also to gain more knowledge of which patients will have benefit of the treatment and who will not.