Ultra-hypofractionated SABR in High risk prostate cancer: preliminary results of a phase II trial
PO-1475
Abstract
Ultra-hypofractionated SABR in High risk prostate cancer: preliminary results of a phase II trial
Authors: Angel Montero1, Karla Rossi2, Ovidio Hernando2, Mercedes Lopez2, Jeannette Valero2, Carmen Cañadillas2, Xin Chen-Zhao2, Raquel Ciervide2, Mariola Garcia-Aranda2, Beatriz Alvarez2, Alejandro Prado3, Rosa Alonso2, Emilio Sanchez2, Marta Izquierdo2, Pedro Fernandez-Leton3, Carmen Rubio2
1HM HOSPITALES, Radiation Oncology, Madrid, Spain; 2HM Hospitales, Radiation Oncology, Madrid, Spain; 3HM Hospitales, Medical Physics, Madrid, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
To evaluate feasibility and tolerance of ultra-hypofractionated (UHF) urethra-sparing SABR in a prospective cohort of high-risk prostate cancer patients
Material and Methods
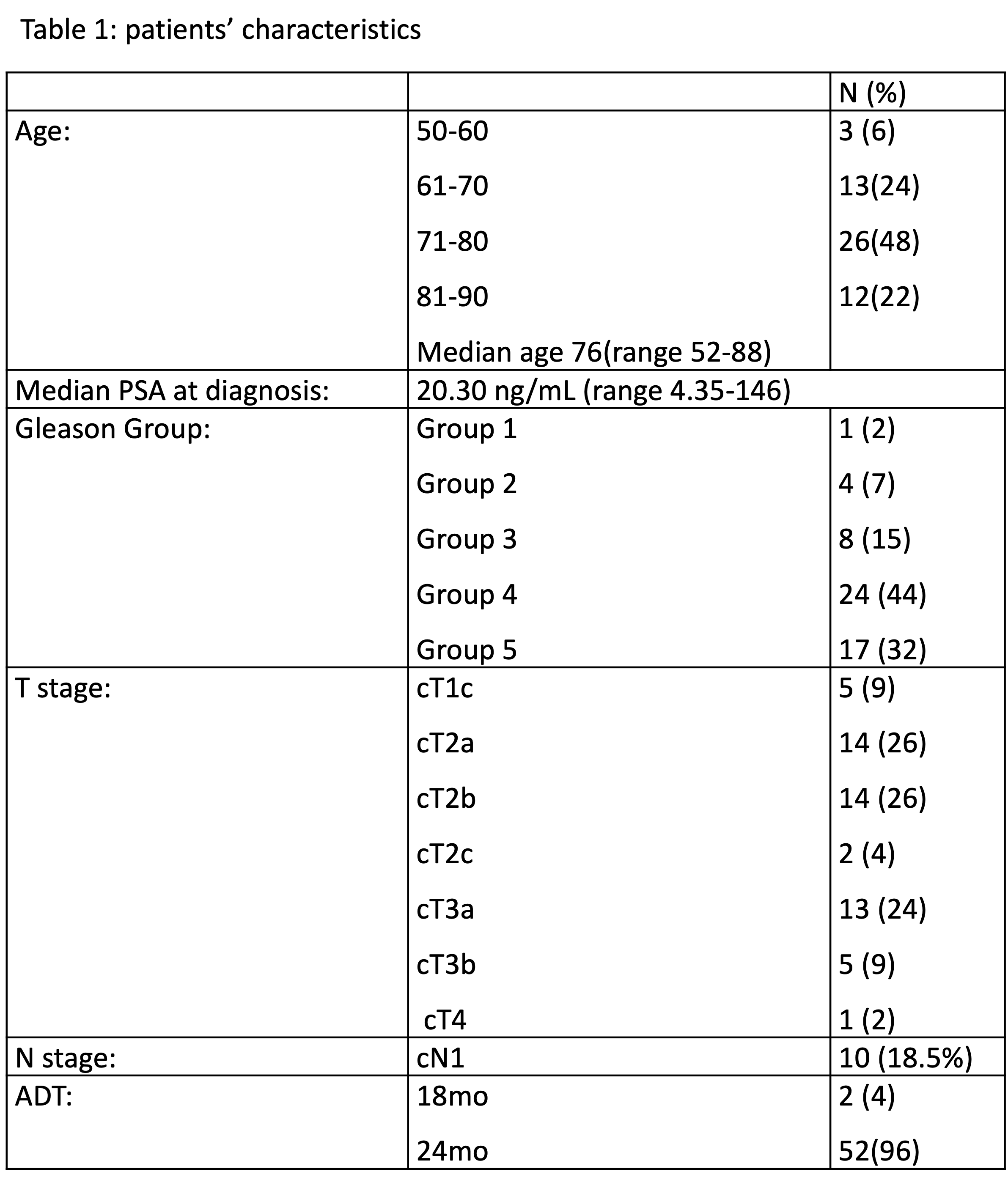
From March 2020 to May 2022, 54p with a median age of 76-yo (52-88) were include in this prospective phase II trial. Patients’ characteristics are detailed in table 1.
All patients underwent VMAT up to a total dose of 40Gy in 5 fractions of 8Gy on every other day (EQD21.5Gy = 109Gy, BED1.5Gy = 253.3Gy) with an urethra-sparing protocol that reduced prescribed dose per fraction to the urethra and the surrounding transitional zone from 8 Gy to 7.2 Gy; 24p (44%) underwent elective nodal pelvic irradiation up to a median dose of 26 Gy in 5 fractions (EQD21.5Gy = 49.8Gy) and 10p (18.5%) with macroscopical pelvic nodal disease received simultaneous integrated boost up to 40 Gy in 5 fractions (Fig.1 shows planning and dose-constraints).
All patients underwent an image-guided-radiotherapy (IGRT) protocol: 33p (61%) with daily cone-beam-CT for positioning and ExacTrac system based upon prostate gold-fiducial markers for intrafraction control, and 21p (39%) by Clarity-4D Monitoring system. Daily immobilization with an endorectal balloon filled-up with 80-100cc air to minimize rectal movements was used. All patients received androgenic deprivation therapy (ADT): 52p for 24 months and 2p for 18 months.Finally, all patients were pre-medicated with alpha-1 receptor antagonist before, during and up to one month after completing SBRT.
Acute a late toxicity were assessed according to RTOG/EORTC and CTCAE v5.0 criteria at one month after the end of SABR every three months during first two years every or six months thereafter and according to in-house follow-up program.
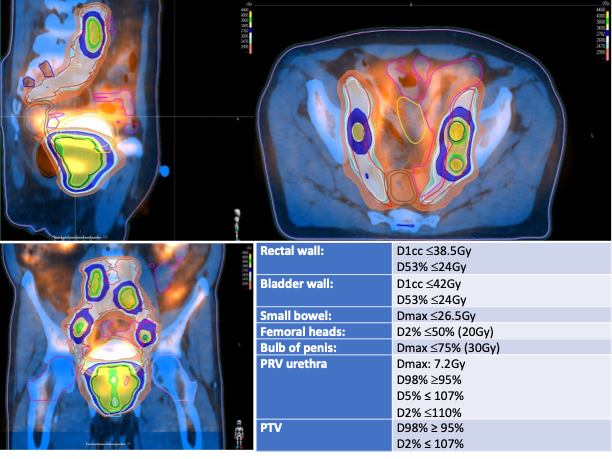
Results
With a median follow-up of 17.8 months (1-43) 98.1% patients are alive and 1p (1.9%) died for non-cancer cause; no patients developed biochemical progression and no distant relapses were observed.
Acute genitourinary (GU) toxicity: 25p (46%) G1, 3p (6%) G2; none G3 toxicity and 26p (48%) had no toxicity. Acute gastrointestinal (GI) toxicity: 5p (9%) G1, 4p (8%) G2; none G3 and 45p (83%) G0.
Late toxicity was assessed in 44p (81%) with a follow up of more than three months. Late-GU adverse-effects: 38p (86%) G0, and 3p (7%) had G1 and G2 respectively but no patients had grade ≥ 3 late GU toxicity. Late-GI adverse-effects: 39p (89%) had no late complications, 3p (7%) G2 and 2p (5%) G3. On follow-up, no patient had persistent symptoms at 12 months post-treatment, since these were adequately resolved.
Conclusion
UHF SABR with a urethra sparing protocol seems to be a feasible and safe option in high risk cancer prostate. However, higher number of treated patients with longer follow-up is necessary to confirm observed results.