PFS Following SBRT for Visceral Oligometastatic Prostate Cancer: A Multicenter Retrospective Study
PO-1466
Abstract
PFS Following SBRT for Visceral Oligometastatic Prostate Cancer: A Multicenter Retrospective Study
Authors: Maximilien Rogé1, Ciro Franzese2, Marta Scorsetti3, Johathan Khalifa4, Corentin Pasquier4, Pierre Blanchard5, Mario Terlizzi5, Emmanuel Seront6, Stéphane Supiot7
1Centre Henri Becquerel, Department of Radiation Oncology, Rouen, France; 2IRCCS Humanitas Research Hospital, Radiotherapy and Radiosurgery Department , Milano, Italy; 3IRCCS Humanitas Research Hospital, Radiotherapy and Radiosurgery Department, Milano, Italy; 4Institut Universitaire du Cancer de Toulouse, Department of radiation Oncology, Toulouse, France; 5Institut Gustave Roussy, Department of radiation Oncology, Villejuif, France; 6Cliniques Universitaires Saint Luc, Department of Medical Oncology, Brussel, Belgium; 7Institut de cancérologie de l'Ouest, Department of Radiation Oncology, Nantes, France
Show Affiliations
Hide Affiliations
Purpose or Objective
Several studies have investigated the role of Stereotactic Body Radiotherapy (SBRT) alone or in combination with systemic treatment in oligo-recurrent prostate cancer (PCa) patients. Published data have shown benefits regarding oncological outcomes with acceptable toxicity (1,2). However, these studies included a very limited number of M1c patients, and no dedicated case series of SBRT in this setting have been reported. Our study aimed to report the oncological outcomes of patients with oligometastatic PCa with visceral metastases treated with SBRT.
Material and Methods
This international retrospective multicenter study included patients with oligometastatic PCa with visceral metastases treated with SBRT between 2014 and 2021. The primary objective was to report progression-free survival (PFS). Secondary objectives included distant metastases-free survival (DMFS), subsequent systemic treatment-free survival, androgen deprivation therapy (ADT)-free survival for patients treated with SBRT alone, local recurrence, and the toxicity rate.
Results
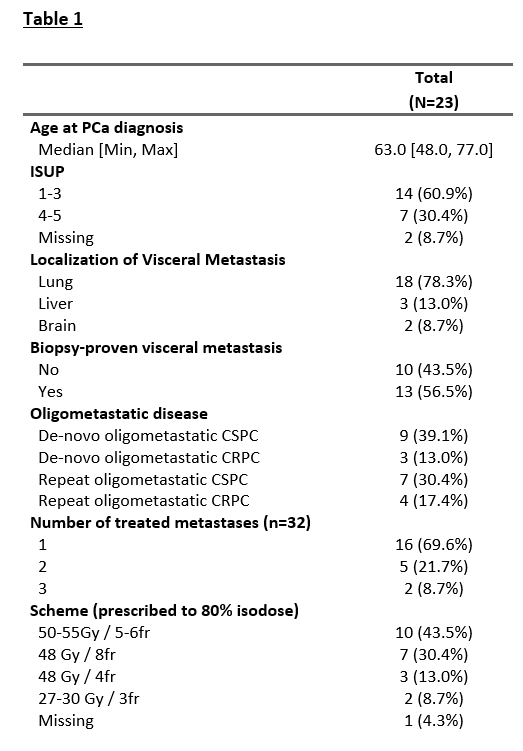
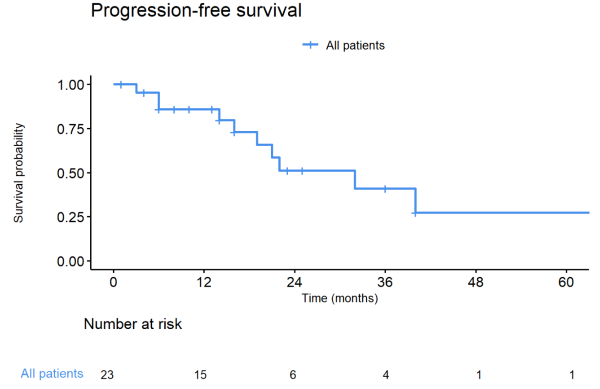
Twenty-three patients were included in our study, with 18 patients (78.3%) with lung metastases, 3 (13.0%) with liver metastases, and 2 (8.7%) with brain metastases treated with SBRT (Table 1). At the time of SBRT, nine patients (55.6%) had a castration-sensitive, and three patients (13.0%) had a castration-resistant de-novo oligometastatic disease. Seven patients (30.4%) had castration-sensitive and 4 patients (17.4%) a castration-resistant repeat oligometastatic disease. SBRT was delivered on 32 metastases, with one treated metastasis for 16 patients (69.9%), two treated metastases for five patients (21.7%), and three treated metastases for two patients (8.7%). The most frequent irradiation schemes, prescribed to 80% isodose, were 50-55 Gy in 5 or 6 fractions (n=10, 43.5%) and 48 Gy in 8 fractions (n=7, 30.4%). After a median follow-up of 22 months (1 – 91) after the end of SBRT, the median PFS, DMFS, and subsequent systemic treatment-free survival were 32 months (figure 1). Only one patient experienced local progression (brain metastases). Three patients died of prostate cancer. SBRT was not associated with any toxicities grade ≥ 2. For the 9 (39.1%) patients treated with SBRT alone, the median ADT-free survival was 22 months.
Conclusion
This is the first study reporting oncological outcomes of oligometastatic PCa M1c patients, specifically with lung metastases treated with SBRT. We showed that SBRT in this setting is a good option without severe toxicities. Because of the lack of specific data on oligometastatic M1c patients, it seems essential to include these patients in prospective studies.