1.5T MR-guided SBRT for Oligometastatic Prostate Cancer: Toxicity and Clinical Outcomes
JING YUAN,
China, Hong Kong Special Administrative Region
PO-1463
Abstract
1.5T MR-guided SBRT for Oligometastatic Prostate Cancer: Toxicity and Clinical Outcomes
Authors: Darren Poon1, Jing Yuan2, Bin Yang3, Oi Lei Wong4, Sin Ting Chiu5, George Chiu5, Kin Yin Cheung3, Siu Ki Yu3
1Hong Kong Sanatorium & Hospital, Comprehensive Oncology Centre, Happy Valley, Hong Kong (SAR) China; 2Hong Kong Sanatorium & Hospital, Research department, Happy Valley, Hong Kong (SAR) China; 3Hong Kong Sanatorium & Hospital, Medical Physics Department, Happy Valley, Hong Kong (SAR) China; 4Hong Kong Sanatorium & Hospital, Research Department, Happy Valley, Hong Kong (SAR) China; 5Hong Kong Sanatorium & Hospital, Department of Radiotherapy, Happy Valley, Hong Kong (SAR) China
Show Affiliations
Hide Affiliations
Purpose or Objective
To prospectively report toxicities and preliminary clinical outcomes of 1.5Tesla (T) MR-guided stereotactic body radiotherapy (MRgSBRT) in patients (pts) with oligometastatic prostate cancer (OMPC).
Material and Methods
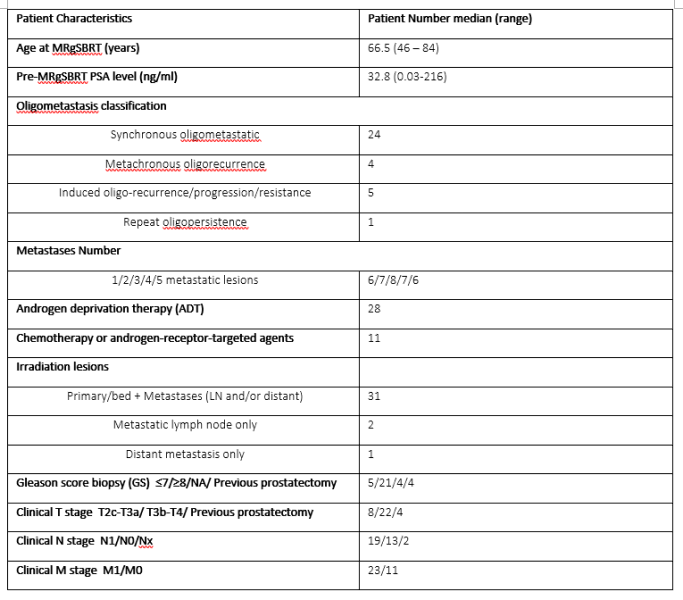
During Apr 2020-Sep 2022, 56 consecutive metastatic PC pts underwent online adaptive MRgSBRT on a 1.5T MR-LINAC plus androgen deprivation therapy (ADT) with or without additional systemic treatments. MRgSBRT was delivered in five fractions (2 fractions/week) of 7.25-8 Gy/fraction to metastases, and of 6.7-8 Gy/fraction to primary/recurrent prostate tumor with daily online adaptation. An isotropic CTV-PTV margin of 5 mm (but 3mm in primary posterior) was applied. Patients were followed and PSA levels were monitored at 1 mo(nth) and then every 3 months. Prostate-specific membrane antigen (PSMA) PET scans were arranged if continuous PSA progressions and/or clinical symptoms were encountered. Toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Overall survival, radiographic progression free survival (rPFS) (by PSMA-PET), and biochemical PFS (bPFS) were estimated using Kaplan-Meier (KM) method. Lesion-based local control (LC) rate was also reported.
Results
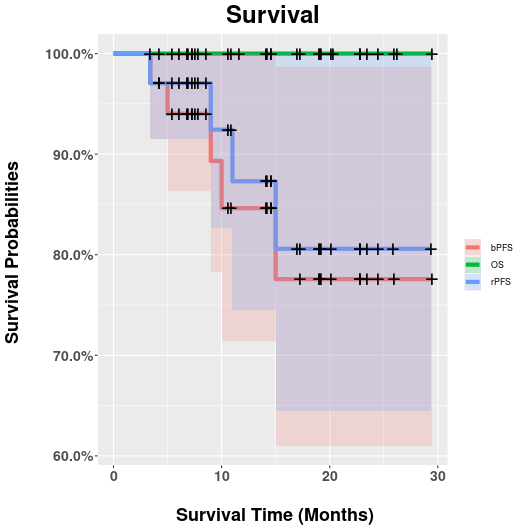
34 (de-novo, n=28; recurrent, n=1; induced, n=5) OMPC pts (median age: 66.5y) with a median 14.2mo FU (3.4mo-29.4mo) were finally included (Table 1). Among them, 27 and 25 pts received ADT only and additional systemic therapy, respectively. All MRgSBRT fractions were successfully delivered with a median duration of 70 minutes (range: 46-104 minutes). Adapt-to-position (ATP) and adapt-to-shape (ATS) were conducted in 120 (71%) and 50 (29%) fractions. In total, 31 primary/recurrent prostate lesions, 47 metastatic lymph nodes (LN), and 29 metastatic bones were irradiated. Characteristics of pts, planning, and treatment were shown in Table 1. Encouraging clinical outcomes were achieved. All pts remained alive during FU. Regarding toxicities, only 3 G2 (1 fatigue; 2 dysuria) in 3 pts but no ≥G3 events were reported. All but 3 bone targets (in 1 pt) responded to treatment and achieved the lesion-based LC rate of 97.2% (104/107) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Four pts showed new metastatic lesions on PSMA-PET. PSA was well controlled in 29 pts and progressed (nadir+2ng/ml) in 5 pts. The estimated OS, rPFS and bPFS at 29-mo were 100% (95%CI: 100%-100%), 81% (95%CI: 65%-100%), and 78% (95%CI: 61%-99%), respectively, as shown in Figure 1.
Conclusion
To our knowledge, this is the first prospective study reporting the clinical outcomes of MRgSBRT for OMPC on a 1.5T MR-LINAC. MRgSBRT was feasible, safe and well tolerated for both primary and metastases synchronously in OMPC pts. The encouraging preliminary clinical outcomes and low toxicities support further exploration of MRgSBRT for OMPC in clinical trials.