Long term urinary function after preoperative radio(chemo)therapy in locally advanced rectal cancer
PO-1398
Abstract
Long term urinary function after preoperative radio(chemo)therapy in locally advanced rectal cancer
Authors: Lisbeth Riber1, Ane L Appelt2, Lars Fokdal1, Heidi S Rønde3, Birgitte Mayland Havelund1
1Vejle Hospital, University Hospital of Southern Denmark, Department of Oncology, Vejle, Denmark; 2University of Leeds, Leeds Institute of Medical Research at St James's, Leeds, United Kingdom; 3Aarhus University Hospital, Danish Centre for Particle Therapy, Aarhus, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
Standard treatment of locally advanced rectal cancer include a multimodality treatment approach with neoadjuvant radio(chemo)therapy to the tumour and elective pelvic lymph nodes followed by total mesorectal excision (TME). Due to the multimodality approach, bladder morbidity may be a point of concern; but detailed reports, including time course of symptoms, are few. We conducted a prospective cohort study (research ethics committee number S-20110021) of long-term patient reported (PRO) and healthcare professional reported toxicity after pre-operative intensity modulated radiotherapy (IMRT) and TME surgery. We here report data on urinary morbidity after treatment.
Material and Methods
A total of 56 patients were included in the period from July 2011 to April 2014. All patients were treated with pre-operative long course radiotherapy, using IMRT optimized to spare bladder & bowel, as well as concomitant Ufteral or Capecitabine. Morbidity (CTCAE v. 4.0, by oncologists or cancer nurses) and PRO (EORTC-QLQ-CR29) was prospectively registered at baseline (BL), end of treatment (EoT), and during each follow up visit for 5-years (for a total of 13 assessments). Proportion of evaluable patients reporting each symptom score at each timepoint was summarized. Change from BL in average cohort scores was evaluated using the wilcoxon signed rank test, and statistically significant changes defined as p<0.05.
Results
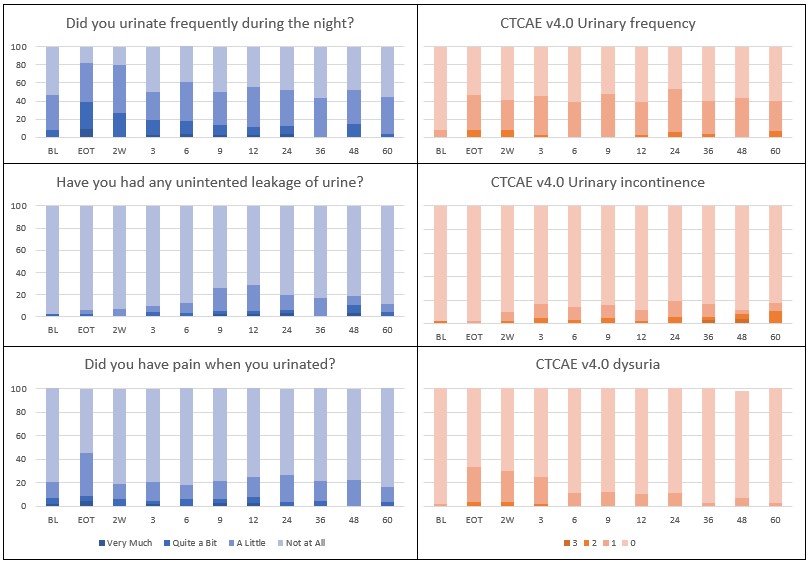
Patient demographics and key bladder dose statistics are reported in Table 1. Median follow up was 92 (9-119) months, with 16 (29%) patients experiencing recurrence during the follow-up period. Urinary symptoms, as reported by patient and healthcare professional, at all timepoints are shown in Figure 1. The most frequent urinary symptoms were frequency, urgency and dysuria; with grade 2 prevalence rates of 3.3%, 3.0% and 0.0% at 3 years, and grade 1 prevalence rates of 36.7%, 27.0% and 3.0% at 3-years. The most frequent PRO endpoint was ‘frequency’, both during the day and during the night; with prevalence rates for ‘Quite a Bit’ of 52.2% and 30.4% at EoT (day: p<0.001 and night: p<0.001) and 19.4% and 8.3% at 12 months (day: p=0.417 and night: p=0.830). No grade 4 and 5 toxicity occurred and only limited grade 3 toxicity occurred regarding urinary incontinence.
Table 1:
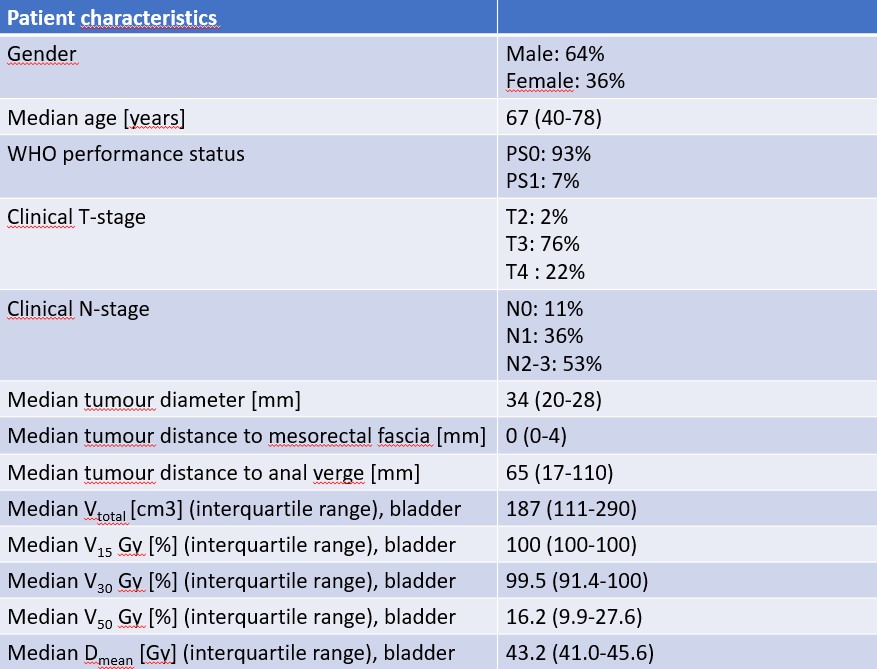
Figure 1:
Conclusion
Physician assessed and patient reported bladder morbidity was generally low after long course radio(chemo)therapy and TME. Severe bladder morbidity was very limited. Mild to moderate urinary frequency and urgency remained elevated during follow-up. Mild to moderate incontinence increased during follow-up. Irritative bladder symptoms (pain, cystitis, dysuria) were reported at EoT but reduced to baseline level during follow-up.