Dose intensification in Locally Advanced Pancreatic Cancer using Robotic SBRT
PO-1391
Abstract
Dose intensification in Locally Advanced Pancreatic Cancer using Robotic SBRT
Authors: marianna valzano1, Mauro Loi1, Pierluigi Bonomo1, Laura Masi2, Raffaella Doro2, Viola Salvestrini2, Marco Banini1, Victoria Lorenzetti1, Ilaria Morelli1, Andrea Romei1, Vanessa Di Cataldo1, Giulio Francolini1, Daniela Greto1, Monica Mangoni1, Gabriele Simontacchi1, Lorenzo Livi1
1Azienda Ospedaliero Universitaria Careggi, Università di Firenze, Radiation Oncology, Firenze, Italy; 2Istituto Fiorentino di Cura e Assistenza (IFCA), CyberKnife Center, Firenze, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy in locally advanced pancreatic cancer (LAPC) has been proposed as a consolidative local treatment after induction chemotherapy in stable disease, or as a primary treatment in patients unfit to either surgery or chemotherapy. It has been proposed that improved local response may be obtained if a Biologically Effective Dose (BED10, assuming α/β=10 Gy) of 100 Gy is delivered to the tumor. We investigated the use of robotic SBRT, with real-time tumor tracking, to a prescribed dose of 50 Gy in 5 fractions (BED10=100 Gy) in a cohort of LAPC patients.
Material and Methods
We conducted a retrospective review of LAPC patients treated with robotic SBRT at our institution from May 2021 to October 2022. Planning aim was to cover ≥93% of the GTV with ≥95%(V47.5) of the target dose (50 Gy in 5 fractions) and 95% of the PTV with 95%(V38) of the target dose (40 Gy in 5 fractions). Dose constraints to Organs at Risk (OAR) were prioritized over target dose objectives. Mandatory constraint for dose-limiting OARs (duodenum, stomach, bowel) were V35≤0.5cc and V25≤10cc. Real-time tumor tracking was implemented following endoscopic ultrasound-guided fiducial marker placement. Toxicity was reported according to CTCAE v 5.1 scale.
Results
Ten patients were included in our analysis. Two patients received exclusive irradiation, while 8 patients underwent SBRT as a local consolidation after obtaining stable disease/partial response following induction chemotherapy with different regimens: Nab-paclitaxel and Gemcitabine for 2 patients; Gemcitabine for 1 patient; FOLFIRINOX for 4 patients; FOLFOX for 1 patient. Median GTV was 45.0 (10.6-81.4) cc. The median number of implanted fiducials for each SBRT treatment was 2 (range 1-2).
Planning aim was achieved in 6 cases. The median GTV (47.5Gy) and PTV(38Gy) coverage was 94.1% (range 79.9 – 100%) and 95,8% (range 92.1– 100%) respectively. Median V35 was 0.14 (range 0.0 – 0.49), 0.10 (range 0.0 – 0.5), and 0.25 (range 0.0 – 0.49) cc for duodenum, stomach and bowel respectively. Median V25 was 4.3 (range 0.0 – 7.97), 1.86 (range 0.0 – 7.78), and 5.63 (range 1.8 – 10.0) cc for duodenum, stomach and bowel respectively (Fig.1).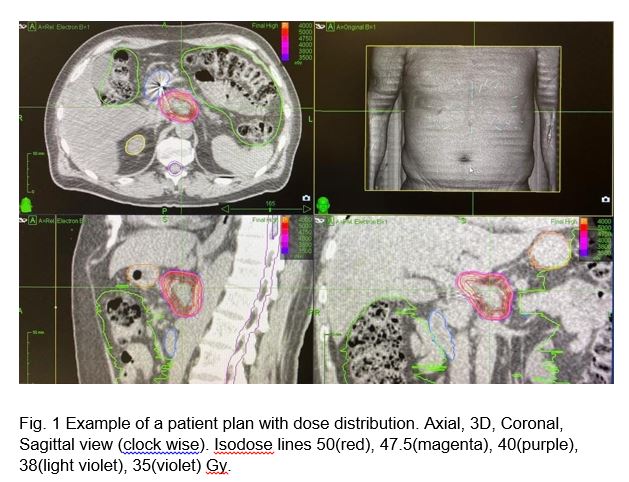
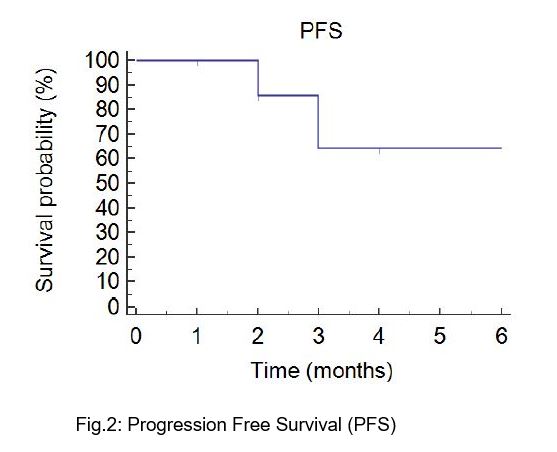
At a median follow-up of 3 (range 3-12) months, no in-field local progression occurred. Median Progression Free Survival was 7 months (Fig.2). No Grade ≥3 treatment-related toxicity was reported. At the time of analysis 2 patients died, 4 experienced out-of-field disease progression, while the remaining patients reported stable disease. Among progressive patients who continued active treatment, metastasis-directed SBRT was performed in 2 cases while one patient resumed systemic therapy.
Conclusion
In patients with LAPC, robotic SBRT using a 50 Gy in 5 fractions schedule was feasible in the majority of patients, with no reported high-grade treatment-related toxicity and no local failure events.