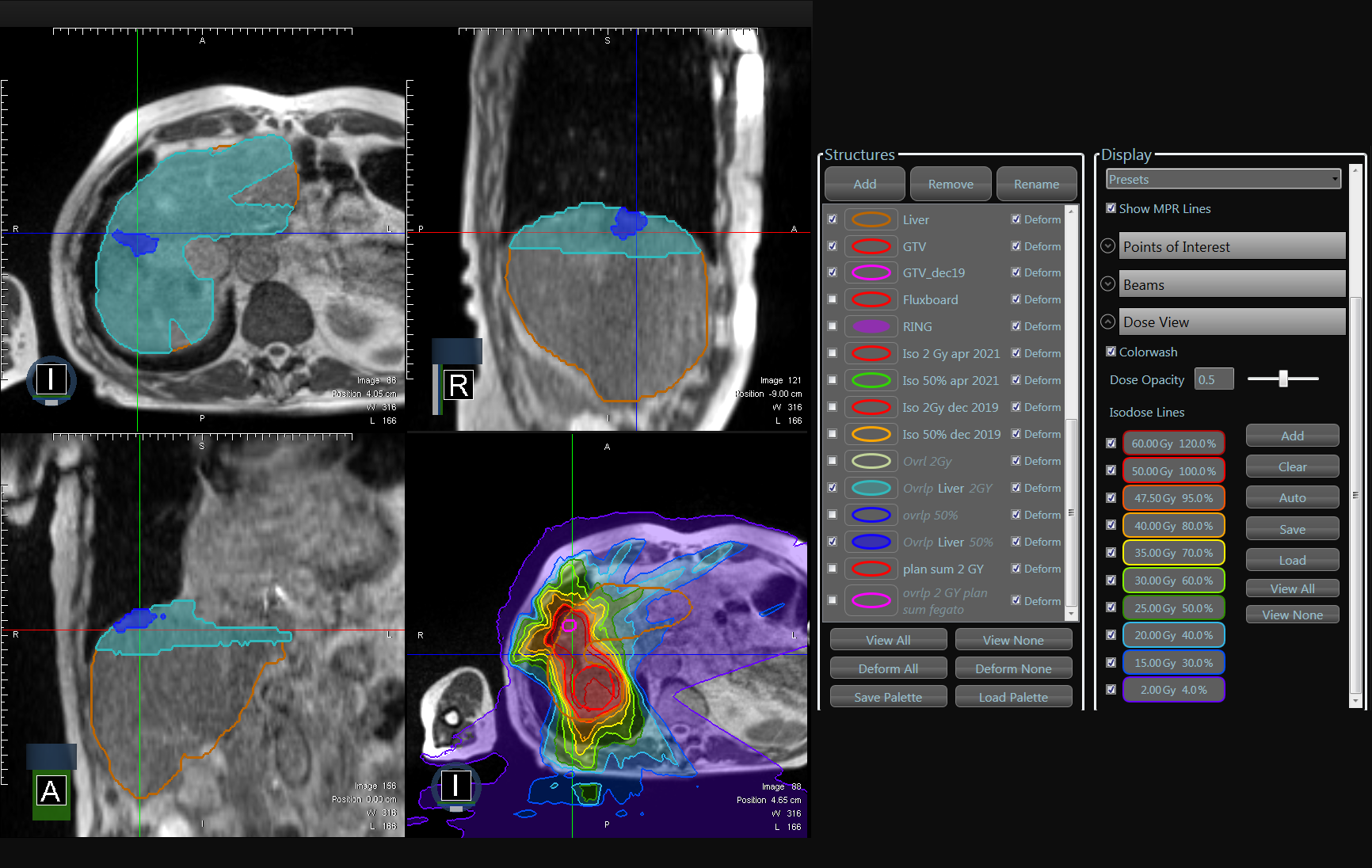
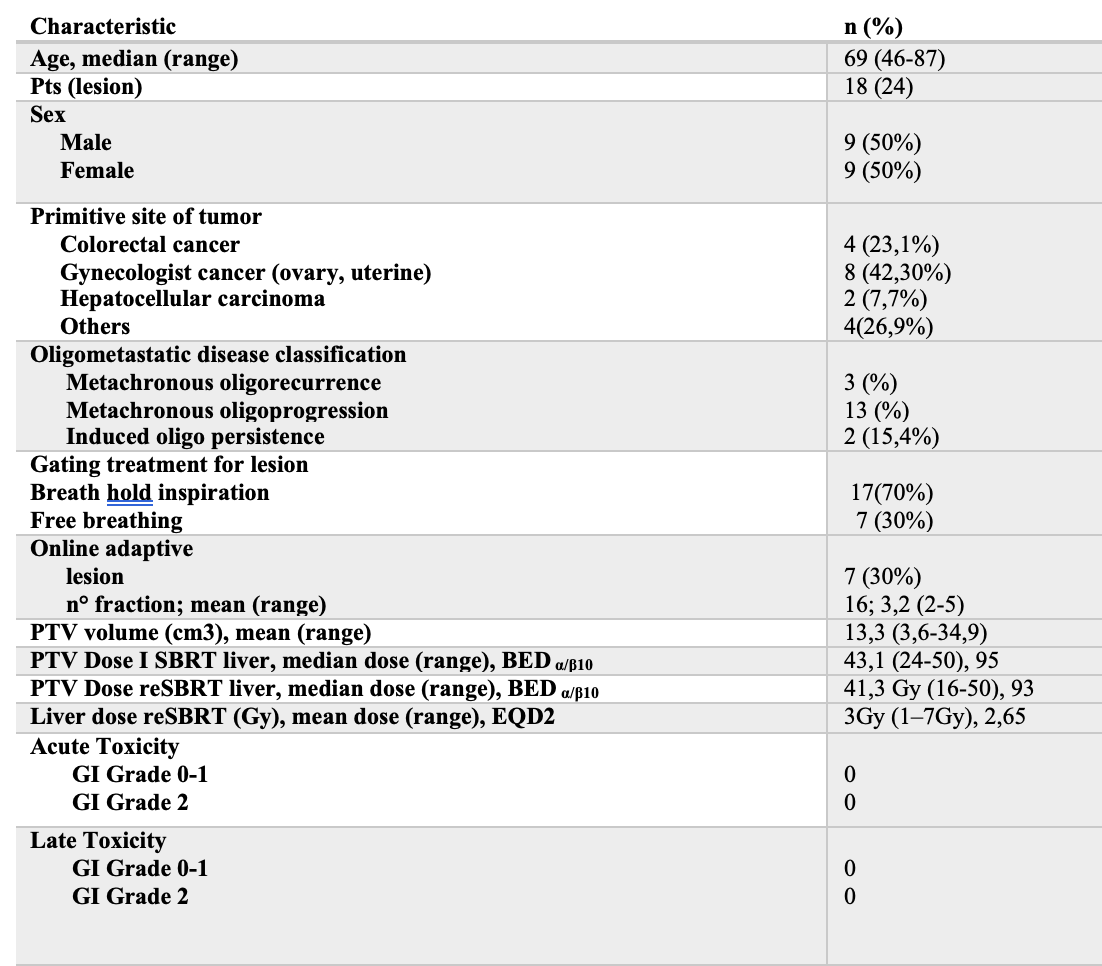
From July 2019 to January 2020 18 pts completed prescribed course of MRIg-reSBRT for 24 metastatic liver lesions, with a mean interval of 8,61 months (range 2-28) from the first SBRT.
First stereotactic treatment was delivered on MR-Linac except for two patients. Table 1 summarize pts characteristic. At least an overlap of the 5% isodoses of prescription dose between the first and second SBRT were reported. For a few cases (22%) there were a minimal overlap between highest isodoses, at least 50% of prescription dose. Fig.1
The mean prescribed dose for the first treatment was 43 Gy (range 24-50Gy, mean BEDα/β10=93).
Instead for reSBRT the mean prescribed dose was 41 Gy (range 16-50Gy, mean BEDα/β10=92).
For 15 pts (79%) the total doses were prescribed to the 80% isodose rather for 4 pts (21%) were prescribed to the dmean covering the 95% of the PTV.
The mean tumor volume (the GTV) was 6,8 cm3 (range 1,5-19,9), with median PTV of 13,4 cm3 (range 3,6-34,9). The mean volume encompassed by the prescription isodose was 11,19 cm3.
Mean PTV D2 and Mean PTV D98 were 51(range 16,5- 67,5) and 41(range 15-59,5), respectively.
The average mean liver dose was 3,9 Gy (range 1-10 Gy; median EQD2 2,9 Gy) and 3,7 Gy (range 1,6-8 Gy; median EQD2 3Gy) for the first SBRT and reSBRT, respectively.
We did not report acute and late toxicities >1 at median follow-up of 10,7 months (range 2-34).
At 1 year OS and PFS was 73,08% and 50% respectively, with an ORR and CB were both 54%.