Isotoxic high-dose SBRT versus CRT for localized pancreatic cancer : a single center evaluation
Martin Manderlier,
Belgium
PO-1378
Abstract
Isotoxic high-dose SBRT versus CRT for localized pancreatic cancer : a single center evaluation
Authors: Martin Manderlier1, Julie Navez2, Matthieu Hein3, Jean-Luc Engelholm4, Jean Closset2, Maria Antonietta Bali5, Dirk Van Gestel1, Luigi Moretti1, Jean-Luc Van Laethem6, Christelle Bouchart1
1HUB Institut Jules Bordet, Department of Radiation Oncology, Brussels, Belgium; 2Hôpital Universitaire Erasme, Department of Hepato-biliary-pancreatic surgery, Brussels, Belgium; 3Université Libre de Bruxelles, Faculty of Medicine, Brussels, Belgium; 4Hopitaux Iris Sud, Department of Radiology, Brussels, Belgium; 5HUB Institut Jules Bordet, Department of Radiology, Brussels, Belgium; 6Hôpital Universitaire Erasme, Department of Gastroenterology, Hepatology and Digestive Oncology, Brussels, Belgium
Show Affiliations
Hide Affiliations
Purpose or Objective
In lack of direct comparative evidence of isotoxic high-dose stereotactic body radiotherapy (iHD-SBRT), we compared the clinical outcomes of patients treated for localized pancreatic ductal adenocarcinoma (PDAC) by iHD-SBRT with those of patients treated with conventional chemoradiotherapy (CRT) in the same tertiary cancer center.
Material and Methods
From January 2006 to January 2021, all consecutive biopsy-proven borderline/locally advanced (BR/LA) patients treated with iHD-SBRT (35Gy in 5 fractions with a simultaneous integrated boost up to 53Gy; January 2018 - January 2021) or conventional CRT (45-60Gy in 25-30 fractions; January 2006 – December 2017) as a primary neoadjuvant or definitive treatment strategy were retrospectively included. In the CRT group, a clinical target volume (CTV) was generated using an expansion of 1 cm from the gross tumour volume (GTV) and completed by the elective nodal regions around the superior mesenteric vessels, portal vein and celiac axis. For the iHD-SBRT group, an internal target volume (ITV) accounting for respiratory motion was created for the GTV and the tumour-vessel interface structure (whole circumference of abdominal vessels in contact with GTV). iHD-SBRT was integrated in a total neoadjuvant strategy, before surgical exploration and after induction chemotherapy by modified FOLFIRINOX for ideally 6 cycles. The median overall survival (mOS) was further evaluated trough uni- and multivariate analyses. The median progression free survival (mPFS) and the 1-year local control (1y-LC) were also reported.
Results
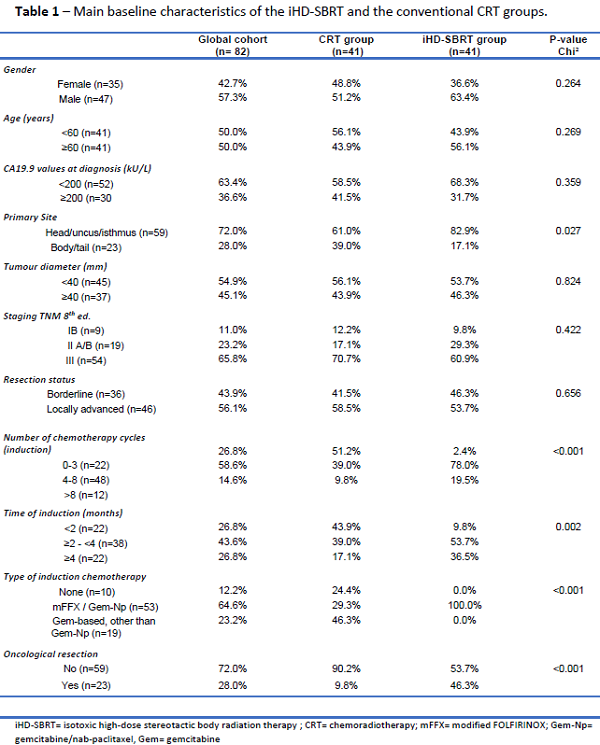
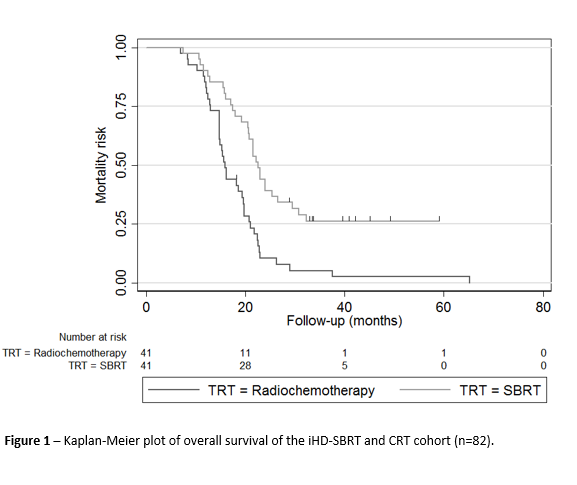
82 patients (41 treated with iHD-SBRT and 41 with CRT) were included. The main baseline characteristics of both groups were comparable. Significant differences in terms of duration and type of induction chemotherapy, and oncological resection rates were identified between the groups (Table1). After a median follow-up of 19.7 months, the mOS (22.5 vs. 15.9 months, p<.001), mPFS (16.7 vs. 11.5 months, p=.011) and 1y-LC (75.8 vs. 39.3%, p=.004) were all in favour of the iHD-SBRT group (Figure 1). Through univariate Cox regression analysis, the following factors were significantly associated with the mortality risk: number and duration of induction chemotherapy cycles, type of induction chemotherapy and radiotherapy received, and oncological resection. A multivariate Cox regression analysis for the mortality risk associated with radiotherapeutic treatments was performed. After adjusting for the main significant confounding factors highlighted during univariates analysis, multivariate analysis demonstrated that unlike CRT, iHD-SBRT was significantly associated with a lower mortality risk for BR/LA PDAC (HR 0.39 [CI95% 0.18 – 0.83], p=.014).
Conclusion
iHD-SBRT is a promising radiotherapeutic option and may offer an improvement in mOS in comparison with conventional CRT for localized PDAC. Further studies are required to confirm the exact role of iHD-SBRT and the optimal therapeutic sequence for the treatment of localized PDAC.