Neoadjuvant radiotherapy after chemotherapy for borderline resectable pancreas cancer
PO-1376
Abstract
Neoadjuvant radiotherapy after chemotherapy for borderline resectable pancreas cancer
Authors: RIE ASSO1, Ian Gerard1, Fabio Cury2, Neil Kopek1
1McGill University Health Centre, Division of Radiation Oncology , Montreal, Canada; 2McGill University Health Centre, Division of Radiation Oncology, Montreal, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
Recent randomized studies of borderline resectable pancreas cancer have shown an increase in overall survival with neoadjuvant therapy when compared to upfront surgery. The optimal neoadjuvant regimen has not been well defined. Achieving an R0 resection poses a particular challenge among borderline resectable patients and has motivated the incorporation of radiotherapy into pre-operative treatment strategies. Hypofractionated radiotherapy (HFRT) has been explored for unresectable pancreas cancer, and reports are largely favourable in terms of tolerability and tumour control rates. However, there is limited published evidence using HFRT in the setting of borderline resectable pancreas cancer. To analyze oncological outcomes and side effects of neoadjuvant chemotherapy followed by HFRT in the management of borderline resectable pancreas cancer.
Material and Methods
This is a single institution retrospective review of patients diagnosed with borderline resectable pancreas cancer treated with neoadjuvant chemotherapy and HFRT followed by surgery. The prescribed dose was 25 to 30Gy in five fractions. Cases treated with exclusively palliative intent or considered unresectable were excluded. Descriptive statistics were used to analyze demographic and disease characteristics. NCI Common Terminology Criteria for Adverse Events were used for toxicity grading. Kaplan-Meier method estimated the clinical outcomes.
Results
A total of 23 patients with pathologically confirmed pancreatic adenocarcinoma were included. The most common prescription dose was 25Gy (n=21) in five fractions. Only two patients received 30Gy. The majority (91.3%) received FOLFIRINOX (4 12 cycles) and 8.6% received gemcitabine-based chemotherapy. The median interval between the end of RT and surgery was 8 weeks (range: 6.4 – 12.1). Most frequent procedure was Whipple’s surgery (56.5%), followed by total (17.3%) and partial (17.3%) pancreatectomy. At the time of surgery, 2 cases were considered unresectable. The median greatest dimension of resected tumours was 2.85 cm. R0 resection status was observed in 18 cases, and 2 cases were microscopically positive (R1). Three cases achieved pathological complete response, 4 minimal residual disease, 4 had partial response (described as residual single cells or rare small groups), and 2 had minimal response. The course of RT was well tolerated: 60.8% did not have any acute side effects. The most common side effect was fatigue (n=7), followed by nausea (n=3), pain, vomiting and abdominal discomfort (n=1 for each). All low grade. The median overall survival (OS) was 20 months. The 3, 6, 12 and 24 month OS was: 95.7%, 87%, 72.4% and 45.5% respectively.
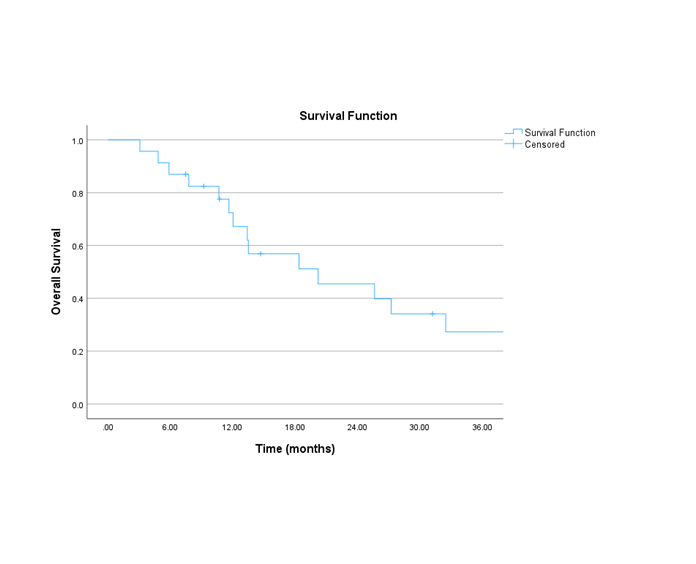
Conclusion
The addition of HFRT after multi-agent chemotherapy as part of a neoadjuvant treatment strategy for borderline resectable pancreatic cancer appears to be very well tolerated and may have contributed to the encouraging R0 resection rate observed in this cohort.