A systematic review of definitive radiotherapy for inoperable, non-metastatic gastric cancer
PO-1375
Abstract
A systematic review of definitive radiotherapy for inoperable, non-metastatic gastric cancer
Authors: Amy Case1, Hayley Hutchings2, Susan Prosser3, Gareth Jenkins4, Tom Crosby5, Richard Adams6, Sarah Gwynne1
1South West Wales Cancer Centre, Clinical Oncology, Swansea, United Kingdom; 2Swansea University , Swansea University Medical School, Swansea, United Kingdom; 3Swansea Bay University Health Board, Singleton Hospital, Swansea, United Kingdom; 4Swansea University, Swansea University Medical School, Swansea, United Kingdom; 5Velindre Cancer Centre, Clinical Oncology, Cardiff, United Kingdom; 6Centre for Trials Research, Cardiff University, Cardiff, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
For patients with inoperable gastric cancer (GC) due to medical co-morbidity, technical inoperability, or patient choice, any treatment is palliative. Inoperable tumours of the gastro-oesophageal junction (GOJ) may be treated with definitive chemoradiotherapy (dCRT), but due to lack of phase 3 data and toxicity concerns, this approach is not widely used in the UK for GC. However, increasing evidence in the neoadjuvant setting suggests gastric RT is tolerable and efficacious. To investigate the potential role of dCRT for inoperable non-metastatic GC, a systematic review (SR) was performed; to our knowledge the first in this context.
Material and Methods
The SR protocol was registered with PROSPERO (reg no.CRD42022297080) and conducted in line with PRISMA standards. Ovid Medline, EMBASE and Cochrane library were searched using relevant MeSH terms, retrieving 10049 records. A manual search of ASCO meeting proceedings for the last 2 years and search of clinicaltrials.gov for gastric RT trials was performed. After duplicate removal and initial screening, 1089 remained for full text review. Following application of well-defined inclusion/exclusion criteria, 2nd reviewer screening, QA and discussion regarding uncertain titles, 9 papers were selected for final analysis (pending final citation chaining).
Results
Since 1998, there have been 3 phase II studies (all single arm), 1 phase I and 5 retrospective case series, totalling 294 (range 8-71) patients. Dose/fractionation is consistent, with 8 of 9 studies delivering between 45Gy/25#-50.4Gy/28#. Two studies report a boost of 5.4Gy/3# to a GTV boost volume (after 45Gy/25# to PTV). RT volumes were inconsistent with no two studies reporting the same target volumes. 3D-CRT was the most common treatment planning modality (n=6) followed by IMRT (n=3). Only 1 study reported IGRT technique, stipulating DIBH, stomach filling protocol and twice weekly CBCT. Chemotherapy regimen differed in every study. Median OS ranged from 11-26.4 months and cCR rate from 8-45% (table). Subgroup analyses in 4 studies suggest improved mOS for patients who achieve complete response (up to 30.7 months). Multivariate analysis by 2 studies show significant benefit of the addition of RT to chemotherapy in this setting. Most common G3/4 toxicities were nausea/vomiting, neutropenia, and lymphopenia. High global quality of life scores were reported by 1 study. Only 1 treatment related death was recorded. The clinicatrials.gov registry revealed 3 active gastric RT trials in this setting, in contrast to 18 neoadjuvant and 9 palliative RT studies.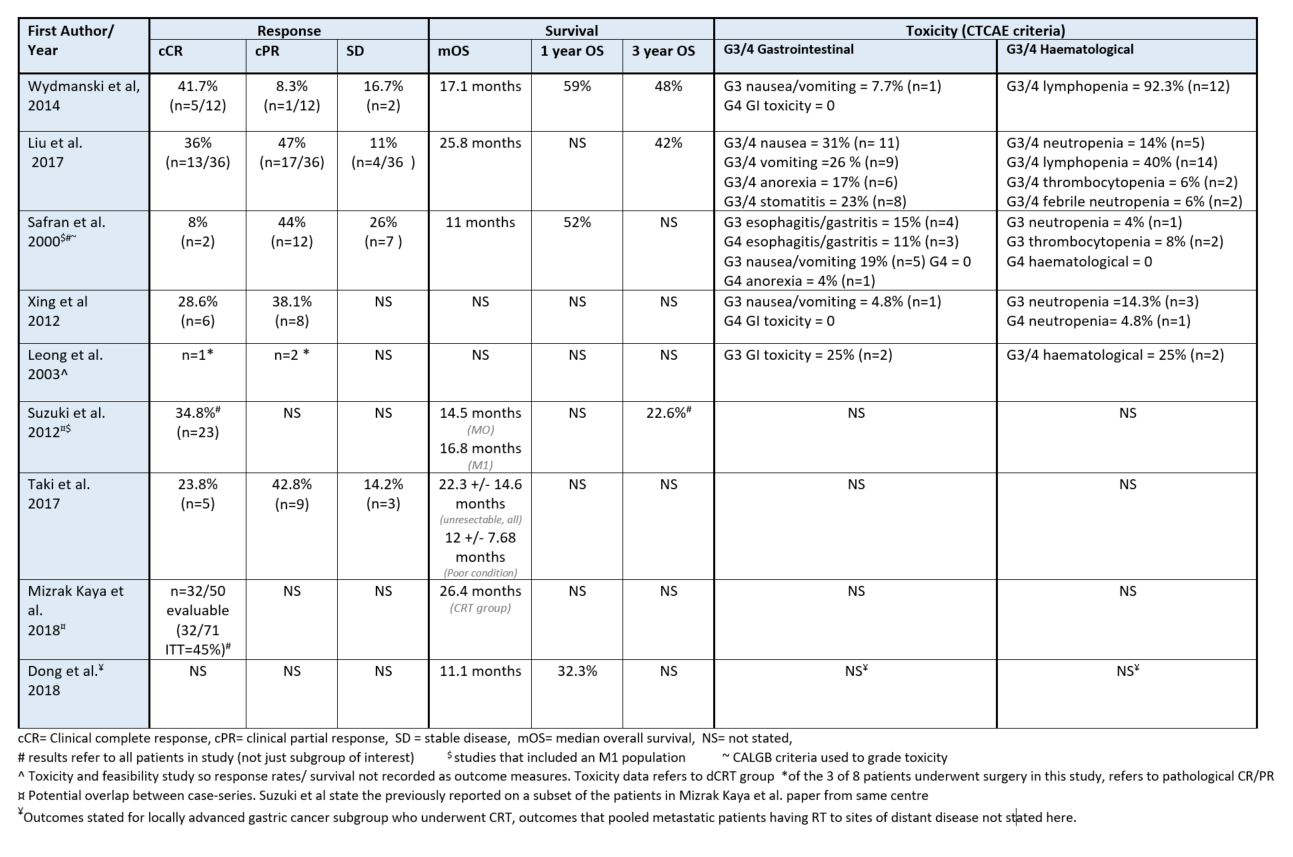
Conclusion
This SR supports a potential role for dCRT for inoperable, non-metastatic GC. In the era of modern RT it is possible technique can be further refined to maximise tumour control whilst minimising toxicity, but target volumes for definitive GC delineation must be refined and standardised. A phase 3 RCT of dCRT combined with optimal systemic therapy, defined RT protocol and RTQA programme is required to further investigate.