Stereotactic Body Radiotherapy in isolated recurrence of pancreatic cancer after radical surgery
Maria Ausilia Teriaca,
Italy
PO-1373
Abstract
Stereotactic Body Radiotherapy in isolated recurrence of pancreatic cancer after radical surgery
Authors: Maria Massaro1, Tiziana Comito1, Maria Ausilia Teriaca1, Ciro Franzese1,2, Luciana Di Cristina1,2, Antonio Marco Marzo1,2, Giacomo Reggiori1, Pasqualina Gallo1, Marta Scorsetti1,2
1IRCCS Humanitas Research Hospital, Radiotherapy and Radiosurgery, Via Manzoni 56, Rozzano, Milan, Italy; 2Humanitas University, Biomedical Sciences, Via Rita Levi Montalcini 4, Pieve Emanuele, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The aim of the study was to assess the efficacy and the tolerance of stereotactic body radiation therapy (SBRT) in patients affected by isolated recurrence of pancreatic cancer after radical surgery, evaluating clinical outcomes and toxicity profile.
Material and Methods
We performed a retrospective analysis on patients with isolated recurrence from resected pancreatic cancer. Prescription dose was 45 Gy in 6 fractions. Primary endpoint was local control (LC), secondary endpoints were distant progression free survival (DPFS), overall survival (OS) and toxicity. Local control was defined according to RECIST criteria. We performed an univariate and a multivariate analysis to evaluate a correlation between endpoints and prognostic factors. Acute and late toxicity was recorded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
Results
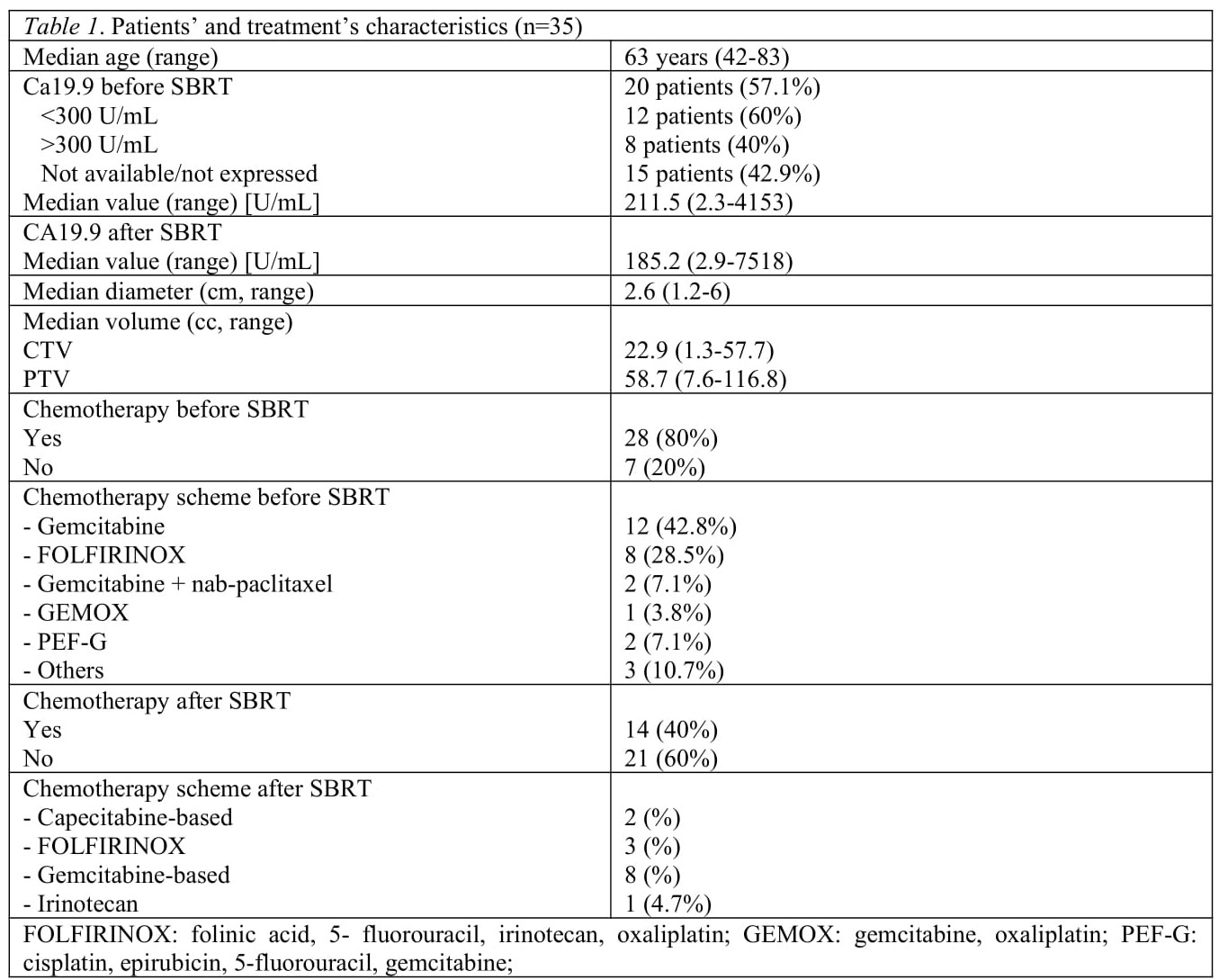
Between 2010 and 2021, 35 patients with isolated local recurrence of resected pancreatic were treated with SBRT. Pancreato-duodenectomy was performed on 25 patients, distal pancreatectomy in 10 cases, all with radical resection (R0). Demographic, clinical and treatment characteristics are shown in Table 1.
Median follow-up was 14 months (5.7 - 94.7). Median LC was 30.2 months. One-, 2- and 3-year LC rate was 67.8% (95%CI 47.9 – 81.4), 62.9% (95%CI 42.2 – 77.9) and 46.6% (95%CI 22.4 – 67.7), respectively. According to RECIST criteria, we observed one patient with complete response (2.8%), 7 partial response cases (20%), 14 patients showed disease stability (40%) and 13 (37.2%) had a local progression. Median DPFS was 9.82 months; 1, 2 and 3-year DPFS rate was 44% (95%CI 26.9 – 59.9), 29.3% (95%CI 13.5 – 47.2) and 29.3% (95%CI 13.5 – 47.2). Six patients (17.1%) were alive at the time of analysis and 2 of them (5.7%) had no evidence of local and metastatic disease. Median OS was 15.1 months and 1-, 2- and 3-year OS rate was 67.6% (95%CI 49.1 – 80.6%), 29.9% (95%CI 15.3 – 46.0) and 19.4% (95%CI 7.7 – 35.0), respectively. We did not observe acute and/or late G3 toxicity.
Conclusion
SBRT has proven to be a promising and safe therapeutic option in these selected long-survivors patients, in terms of good local control rate and low toxicity profile.