A simplified method for delineation of target volumes in 4D oesophageal cancer radiotherapy
Sarah gwynne,
United Kingdom
PO-1371
Abstract
A simplified method for delineation of target volumes in 4D oesophageal cancer radiotherapy
Authors: Geraint Lewis1, Owen Nicholas2, Sarah Gwynne2,5, Tom Crosby3, Alok Chand3, Betsan Thomas3, Lucy Wills1, Ganesh Radhakrishna4
1Velindre Cancer Centre, Medical Physics, Cardiff, United Kingdom; 2South-West Wales Cancer Centre, Clinical Oncology, Swansea, United Kingdom; 3Velindre Cancer Centre, Clinical Oncology, Cardiff, United Kingdom; 4The Christie, Clinical Oncology, Manchester, United Kingdom; 5Swansea University, Medical School, Swansea, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
The guidance for delineation of target volumes for oesophageal cancers being treated using 4DCT within the SCOPE2 trial was updated in an effort to streamline the outlining process. The streamlined method reduces the editing for OARs and ITV creation to a single breathing phase, rather than three. The ITV is then reviewed on all breathing phases to ensure coverage is achieved. This sub-study was designed to investigate whether 4D outlining requirements can be adapted in this way to reduce the demands on clinicians’ time, whilst maintaining robustness in the final target volumes.
Material and Methods
Three anonymised 4DCT datasets were distributed to a group of 6 independent observers with experience in outlining target volumes for oesophageal radiotherapy. In each case, the GTV was provided for the reference, maximum inhalation and maximum exhalation breathing phases. Each participant was asked to outline 1 case using both the existing 4DCT delineation guidance published in the SCOPE2 Radiotherapy Planning Guidance Document (RPGD) and the streamlined guidance.
Each of the cases were reviewed by an experienced consultant clinical oncologist to determine whether the outlining protocol had been followed correctly. The Jaccard similarity index (JCI) and geometric miss index (GMI) were used to quantify the similarity of the ITV and PTV for both delineation methods.
Results
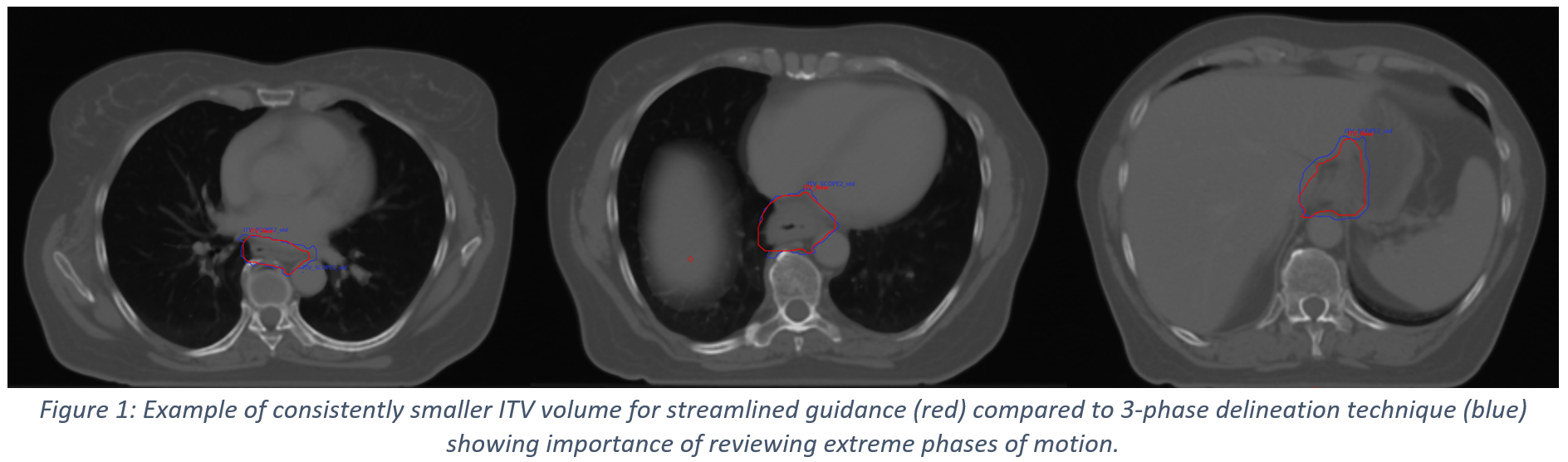
All submitted volumes were found to be either acceptable as per protocol or acceptable with variation for entry into the SCOPE2 trial. Errors related to editing of clinical target volumes for OARs and CTVA length unrelated to protocol accounted for some of the differences observed in the submitted volumes. In one case, the ITV volume was consistently smaller as a result of following the updated guidance, due to extremes of the respiratory cycle not being adequately covered (Figure 1).
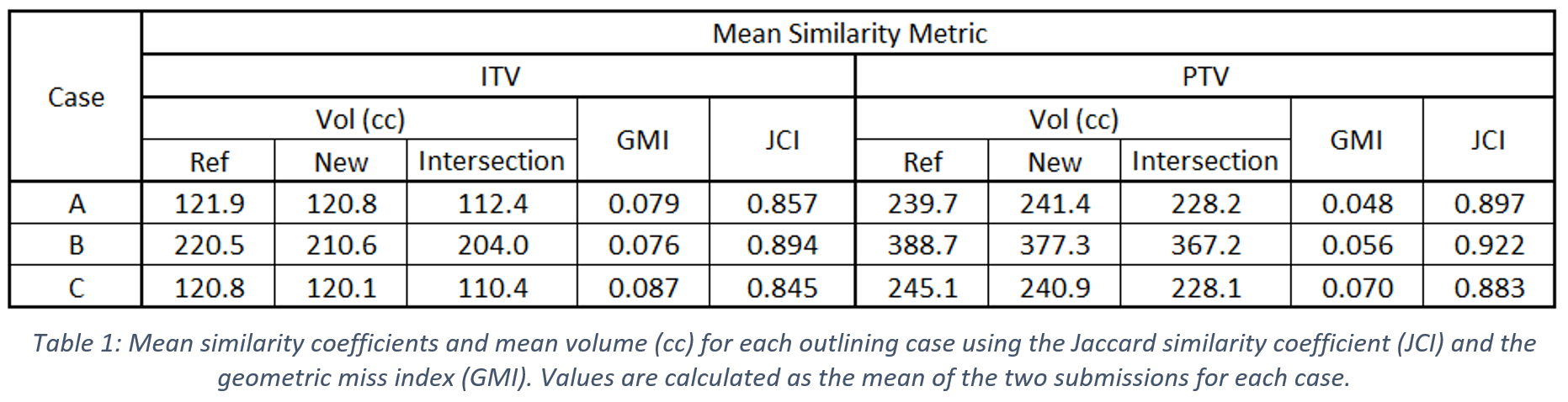
Calculated values of the mean JCI and GMI for each case, as summarised in Table 1, show good agreement between the target volumes produced by following both the original and streamlined outlining methods.
Conclusion
The streamlined 4D outlining methodology produced clinically acceptable target volumes, which agreed well with the existing 3-phase delineation methodology published in the SCOPE2 RGPD. This shows that the streamlined methodology maintains robustness in target volume delineation while reducing the demands on clinicians’ time. The issues observed for one case B submission, where the extremes of breathing motion were not fully-accounted for, resulting in a consistently smaller ITV (Figure 1) highlights the need for careful review of the ITV on all breathing phases.
The streamlined process will be introduced into the SCOPE2 RPGD as an amendment, offering an alternative for trial centres wishing to treat using 4DCT within the trial. Timely-retrospective reviews will be carried out for the first cases for centres switching to the new method to guarantee the suitability of the target volumes.