Albumin-Bilirubin (ALBI) score predicts hepatotoxicity after stereotactic ablative radiation therapy
Ji Hyeon Joo,
Korea Republic of
PO-1368
Abstract
Albumin-Bilirubin (ALBI) score predicts hepatotoxicity after stereotactic ablative radiation therapy
Authors: Ji Hyeon Joo1, Wontaek Kim1, Jiho Nam2, Yongkan Ki1, Donghyun Kim1, Dahl Park2, Hosang Jeon3, Dong Woon Kim3, Jongmoo Park4
1Pusan National University School of Medicine, Department of Radiation Oncology, Yangsan-si, Korea Republic of; 2Pusan National University Hospital, Department of Radiation Oncology, Busan, Korea Republic of; 3Pusan National University Yangsan Hospital, Department of Radiation Oncology, Yangsan-si, Korea Republic of; 4School of Medicine, Kyungpook National University , Department of Radiation Oncology, Daegu, Korea Republic of
Show Affiliations
Hide Affiliations
Purpose or Objective
Unlike other cancer, assessment of underlying liver function plays a key role in predicting treatment outcomes of hepatocellular carcinoma (HCC). As an alternative to Child-Turcotte-Pugh (CTP) classification, a measure of liver function based on albumin and bilirubin has been proposed, the albumin-bilirubin (ALBI) score. We evaluated prognostic performance of ALBI score and determined optimal cutoff point as a potential predictor of hepatotoxicity after stereotactic body radiation therapy (SBRT).
Material and Methods
We retrospectively evaluated 121 HCC cases treated between 2018 and 2020. Raw ALBI scores and CTP scores were calculated. The occurrence of hepatotoxicity was defined as post-SBRT CTP score increase ≥ 2. To compare ALBI and CTP scores, receiver operating characteristic (ROC) curves were calculated and DeLong’s test was used for statistical analysis. The optimal cutoff of ALBI score was calculated. By mean radiation dose to the whole liver, patients were subgrouped and tested.
Results
Median follow-up time was 15.5 months. There were 93 patients of CTP score 5, 19 of CTP 6, 4 of CTP 7, and 5 of CTP 8. Median ALBI score was -2.6 ± 0.4. Hepatotoxicity occurred in 7 patients (5%). Both baseline CTP score and ALBI score predicted the post-SBRT CTP score change. Correlation coefficient was 0.316 for CTP score (P<0.001) and 0.336 for ALBI score (P<0.001). The area under ROC curve predicting hepatotoxicity for ALBI score was 0.77, and for CTP score was 0.71 (P = 0.5019, fig. 1). The optimal cutoff value of the ALBI score was -2.47, with a sensitivity of 85.7% and a specificity of 71.1%. Of the patients with ALBI score >-2.47, 15.38% (6 of 39) developed toxicity, compared to 1.22% (1 of 82) in patients with score ≤-2.47. In the multivariable analysis, ALBI score and PTV were significant factors (odds ratio [OR] of 29.51 for ALBI score, P = 0.003; OR of 1.05 for PTV, P = 0.004). Patients were divided into the following four groups based on ALBI score of 2.47 and PTV of 65 mL; Group 1, ALBI score≤-2.47 & PTV<65mL; Group 2, ALBI score≤-2.47 & PTV≥65; Group 3, ALBI score>-2.47 & PTV<65; Group 4, ALBI score>-2.47 & PTV≥65. The incidence of hepatotoxicity in groups 1, 2, 3, and 4 was 1.47% (1 of 68), 6.25% (1 of 16), 6.25% (2 of 32), and 60% (3 of 5), respectively (P<0.001). In subgroup analysis, area under curve was larger in ALBI than CTP when mean liver dose was higher than 9 Gy, 11 Gy, 12 Gy.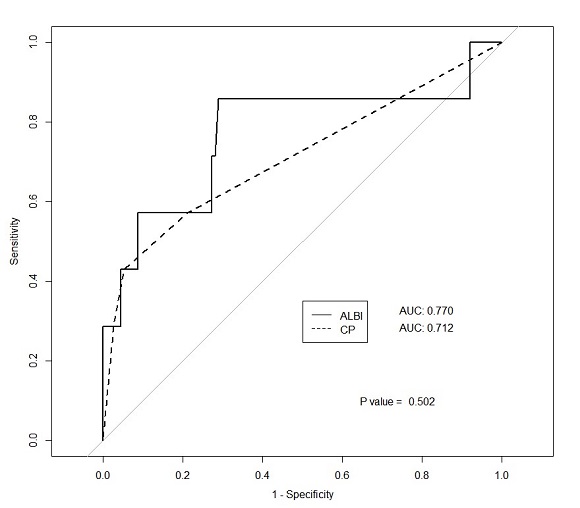
Conclusion
Baseline ALBI score was identified as an independent prognostic factor for hepatotoxicity. The optimal ALBI cutoff value to predict CTP increase was -2.47, which is higher than widely accepted reference point for ALBI grade 1 and 2, -2.60. The higher the MLD, the greater the predictive power of the ALBI score compared to the CTP score.