Lutetium-177 Dotatate peptide receptor radionuclide therapy outcomes for neuroendocrine tumours
Mohammed AlHilali,
United Kingdom
PO-1365
Abstract
Lutetium-177 Dotatate peptide receptor radionuclide therapy outcomes for neuroendocrine tumours
Authors: Mohammed AlHilali1, James Carberry1, Angela Challis2, Philip Atherton1
1The Newcastle upon Tyne Hospitals NHS Foundation Trust, Northern Centre for Cancer Care, Newcastle upon Tyne, United Kingdom; 2The Newcastle upon Tyne Hospitals NHS Foundation Trust, Nuclear Medicine, Newcastle upon Tyne, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Peptide receptor radionuclide therapy (PRRT) is a molecular radiotherapeutic modality in which ligands for somatostatin receptors are attached to therapeutic radionuclides to target neuroendocrine tumour cells. The Neuroendocrine Tumours Therapy (NETTER-1) trial [1] demonstrated 11.7 month difference in median overall survival (OS) benefit with Lutetium-177 Dotatate treatment (Median OS 48.0 months) versus high-dose long-acting octreotide (Median OS 36.3 months) in patients with advanced midgut neuroendocrine tumours (NETs). This might be clinically relevant despite final overall survival not reaching statistical significance. We designed our audit to compare our cancer centre’s practice and outcome with the National Institute for Health and Care Excellence (NICE) guidance [2] and the NETTER-1 trial findings.
Material and Methods
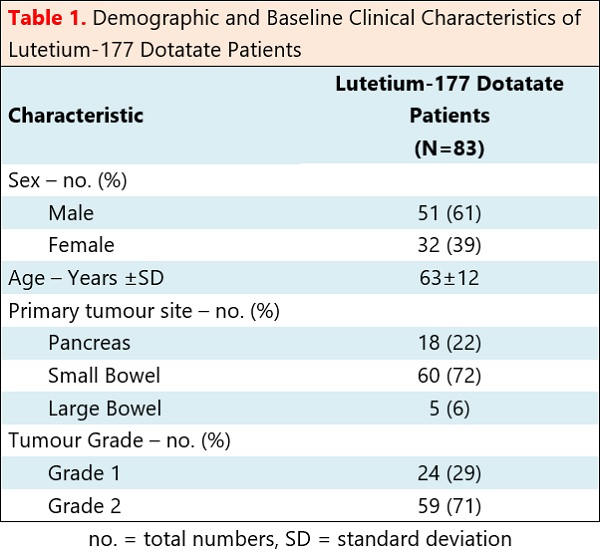
The data was collected from 83 patients who had well-differentiated, metastatic, gastroenteropancreatic neuroendocrine tumours that had started receiving Lutetium-177 Dotatate between August 2019 and September 2022 in the North East and North Cumbria regions of England. The primary outcome measure was overall survival.
Results
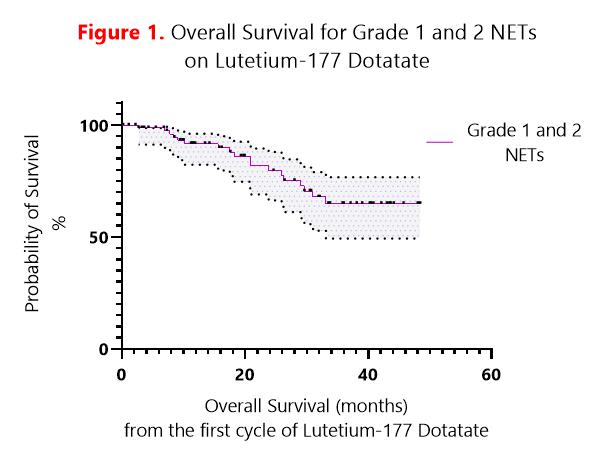
All patients met NICE eligibility criteria for Lutetium-177 Dotatate treatment. Twenty-four patients had grade 1 NETs. Fifty-nine patients had grade 2 NETs. Median OS for grades 1 and 2 NETs was not reached with 64.9% of patients alive at 33.1 months (Figure 1). Median OS for grade 1 NETs was not reached with 72.7% of patients alive at 33.1 months. Median OS for grade 2 NETs was not reached with 61.4% of patients alive at 31 months. Eighteen patients had pancreatic primaries. Sixty-five patients had gastrointestinal primaries. There was no significant difference in survival between pancreatic and gastrointestinal NETs. Nine patients stopped before completing all cycles of treatment due to disease progression or toxicity. Three patients stopped due to mid-treatment disease progression. Two patients stopped due to pancytopenia, 2 due to thrombocytopenia, 1 due to congestive enteropathy and 1 due to renal toxicity.
Conclusion
The audit confirmed our cancer centre follows the available national guidelines. Its clinical outcome is comparable with that of the NETTER-1 trial. Treatment was well tolerated, with most patients completing all treatment cycles. Further areas of sub-set analysis are underway.
References:
1. Strosberg J.R., et al. (2021) 177Lu-Dotatate plus long-acting octreotide versus high dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:1752–1763. doi: 10.1016/S1470-2045(21)00572-6.
2. National Institute for Health and Care Excellence (NICE) (2018) Lutetium (177Lu) oxodotreotide for treating unresectable or metastatic neuroendocrine tumours. Technology appraisal guidance TA539. Available at: www.nice.org.uk/guidance/ta539 (Accessed 25 October 2022).