Liver oligometastatic disease in metastatic gastric cancer patients: a population-based cohort study
Tiuri Kroese,
Switzerland
PO-1361
Abstract
Liver oligometastatic disease in metastatic gastric cancer patients: a population-based cohort study
Authors: Tiuri Kroese1, Yuko Takahashi2, Florian Lordick3, Peter van Rossum4, Jelle Ruurda5, Sjoerd Lagarde6, Richard van Hillegersberg5, Rob Verhoeven7, Hanneke van Laarhoven8
1University Hospital Zurich, Radiation Oncology, Zurich, Switzerland; 2Okayama University Hospital, Medical Oncology, Okayama, Japan; 3University Hospital Leipzig, Medical Oncology, Leipzig, Germany; 4University Medical Center Utrecht, Radiation Oncology, Utrecht, The Netherlands; 5University Medical Center Utrecht, Surgery, Utrecht, The Netherlands; 6Erasmus Medical Center, Surgery, Rotterdam, The Netherlands; 7Netherlands Comprehensive Cancer Organization, Research & Development, Utrecht, The Netherlands; 8Amsterdam University Medical Centers, Medical Oncology, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Local treatment improves outcomes for oligometastatic disease (OMD, i.e. an intermediate state between locoregional and widespread disseminated disease). This population-based cohort study analyzed treatment, overall survival (OS), and independent prognostic factors for OS in gastric cancer patients with liver metastases.
Material and Methods
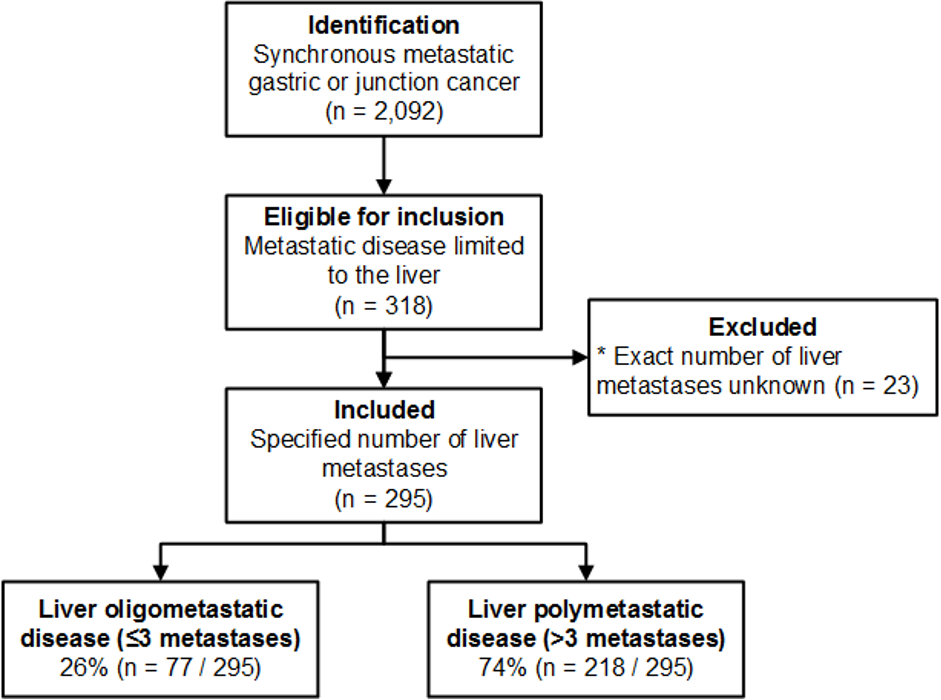
Between 2015 and 2017, patients with synchronous metastatic gastric or gastroesophageal junction adenocarcinoma limited to the liver were included from the prospectively maintained population-based Netherlands Cancer Registry. Liver oligometastatic disease (OMD) was defined as ≤3 liver metastases. The primary outcome was OS. Independent prognostic factors for OS were analyzed using multivariable Cox regression analysis.
Results
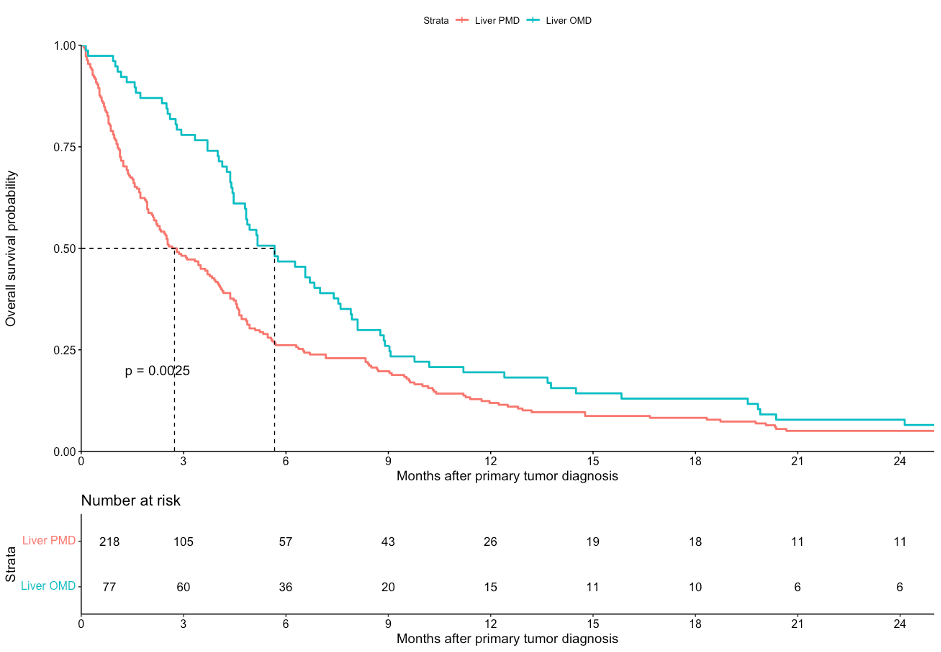
A total 295 patients with metastases limited to the liver were included. The primary tumor was resected in 4 patients (1.4%). Treatment for liver metastases consisted of chemotherapy alone (28.1%), trastuzumab plus chemotherapy (4.7%), surgery (1.0%), or best supportive care (67.5%). Median OS across all included patients was 4.0 months (95% confidence interval [CI]: 3.1-4.5). Liver OMD was detected in 77 patients (26%). Treatment for liver OMD consisted of chemotherapy alone (24.6%), trastuzumab plus chemotherapy (5.2%), surgery (3.9%), or best supportive care (67.5%). Median OS among patients with liver OMD was 5.7 months (95% CI: 4.8-7.5). Better OS was independently associated with liver OMD (hazard ratio [HR] 0.66, 95% CI: 0.50-0.87), trastuzumab (HR 0.41, 95% CI: 0.23-0.72) but not with triplet compared with doublet chemotherapy (HR 0.94, 95% CI: 0.57-2.87). Worse OS was independently associated with unknown nodal stage versus cN0 (HR 1.74, 95% CI: 1.17-2.60), diffuse-type versus intestinal-type adenocarcinoma (HR 2.06, 95% CI: 1.32-3.20), and monotherapy or best supportive care versus doublet chemotherapy (HR 1.72, 95% CI: 1.03-2.87 and HR 3.61, 95% CI: 2.55-5.10, respectively).
Conclusion
In this population-based study, liver OMD was detected in 26% of patients. Liver OMD and trastuzumab treatment were independently associated with better OS while triplet as compared with doublet chemotherapy was not. OS among patients with liver OMD nevertheless remained poor. The concept of OMD and the benefit of resection of liver OMD may still have been relatively unknown in this disease type during the study inclusion years.