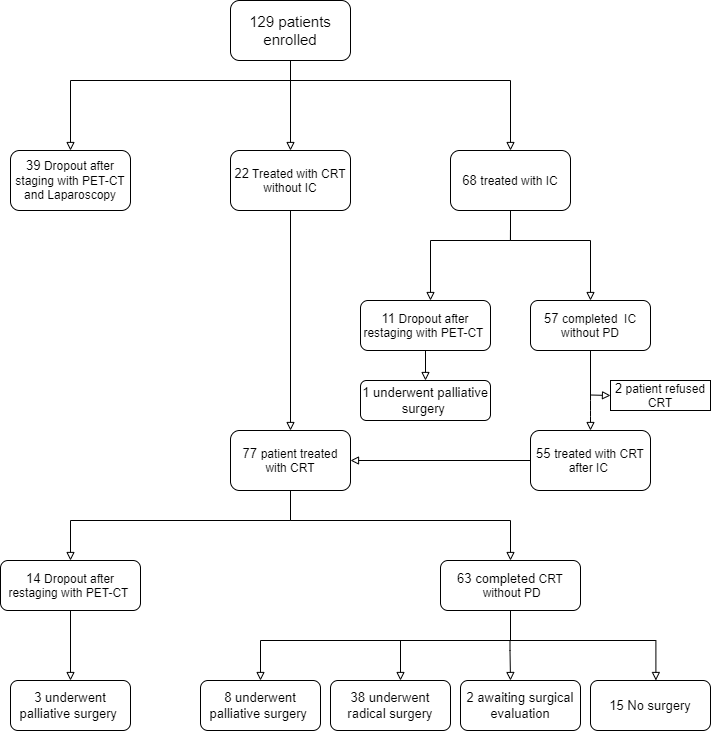
Of the 129 eligible patients (69 men, 60 women), median age was 64 years (range, 36–75 years). According to the results of the pre-treatment workup, thirty-nine patients (30.2%) had metastatic disease and were therefore excluded from the protocols. Sixty-eight patients who underwent IC (Gem-Ox or FOLFIRINOX schemes) were evaluated for clinical response after 2 months by using CT scan and PET-CT scan. Eleven patients (16%) experienced disease progression; two patients refused to continue the protocols. Overall, seventy-seven (59.7%) patients received concomitant CRT and were evaluated for clinical response (Figure 1). The median follow-up for all patients was 21 months (range, 5 to 132 months). Thirty-eight patients (60%) underwent surgical radical resection. R0 resection was achieved in 37 of the 38 resected patients (97.4%). Two patients died due to perioperative complications. For the 77 patients undergoing CRT, the median PFS was 13 months ((95% CI, 8 to 20). One-year, 2-yr and 3-yr PFS were 56%, 32%, 22%, respectively. One-year, 2-yr and 3-yr LPFS were 83%, 68% and 60%, respectively. One-year, 2-yr and 3-yr MFS were 65%, 40%, 30%, respectively. Median OS was 17.5 months (95% CI, 12.1 to 22.8). One-year, 2-yr and 3-yr OS were 80%, 37% and 29%, respectively. Patients who underwent resection had a significantly longer median OS compared with non resected patients (37.6 months vs 13 months, p < 0.001). The median PFS for resected patients was 22.5 months compared with 9.5 months for non resected patients (p< 0.001). The median OS and PFS for patients treated by upfront CRT were 14.6 and 9.9 months compared with 19.2 and 17.8 months for patients treated by IC followed by CRT (p<0.05).
Figure 1: Study flowchart