Predictors of outcomes in patients with NSCLC treated with chemoradiotherapy & immunotherapy.
Ashley Horne,
United Kingdom
PO-1310
Abstract
Predictors of outcomes in patients with NSCLC treated with chemoradiotherapy & immunotherapy.
Authors: Ashley Horne1, Jane Shortall2, Alan Mcwilliam2, Corinne Faivre-Finn1
1The Christie NHS Foundation Trust, Radiotherapy Related Research, Manchester, United Kingdom; 2University of Manchester, Radiotherapy Related Research, Manchester, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
The overall survival of patients with stage III NSCLC has improved with the introduction of consolidation IO following concurrent CRT. Despite this, many patients go on to relapse and around 50% of patients will die of their disease within 4 years of diagnosis.
Biomarkers that more accurately prognosticate patients could be clinically informative and help personalise treatment and follow-up for individual patients. Robust biomarkers based on routinely collected data and blood tests are appealing as they are cost-effective and widely available.
We investigated whether routinely collected standard of care blood tests (and ratios based on them, such as neutrophil/lymphocyte ratio) and clinical variables could be used to predict clinical outcomes in patients with locally advanced NSCLC who received concurrent CRT followed by consolidation IO.
Material and Methods
Data, including clinical variables and routine blood tests results was collected for 78 patients with locally-advanced NSCLC treated with concurrent CRT (60-66Gy in 30-33 fractions with concurrent cisplatin-etoposide or carboplatin-taxol) and consolidation IO (durvalumab) between September/2018 and February/2022. Blood tests were taken prior to CRT and prior to IO (considered up to 1 week prior to commencement of therapy). Primary endpoint was progression free survival (PFS).
Univariable and multivariable Cox proportional hazards models were produced. Clinical and blood variables were selected via bootstrapping and elastic net LASSO analysis. The Akaike Information Criteria (AIC) was used to compare the performance of models not including and including selected blood variables.
Results
All patients had an ECOG PS 0-1, Clinical Frailty Index score of 0-4 and ACE Comorbidity Score of 0-3.
With a median follow-up of 25.5 months, the median PFS was 14.2 months.
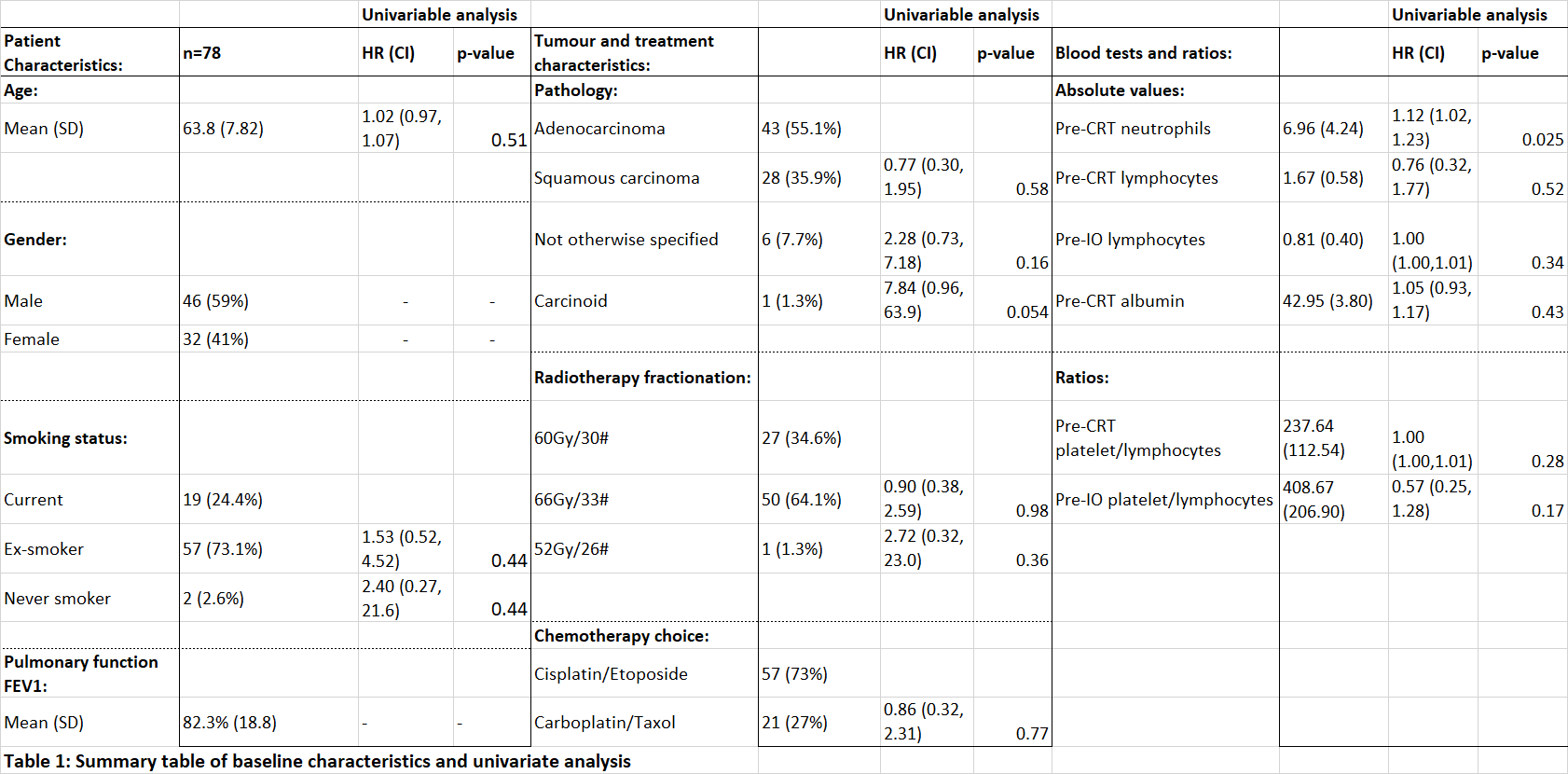
Table 1 summaries key results from the univariable analysis and baseline characteristics. In univariable analysis, only pre-chemoradiotherapy neutrophils were significant for PFS (HR=1.12, 95%CI 1.01 - 1.23; p=0.025). No other blood or clinical variable demonstrated statistical significance.
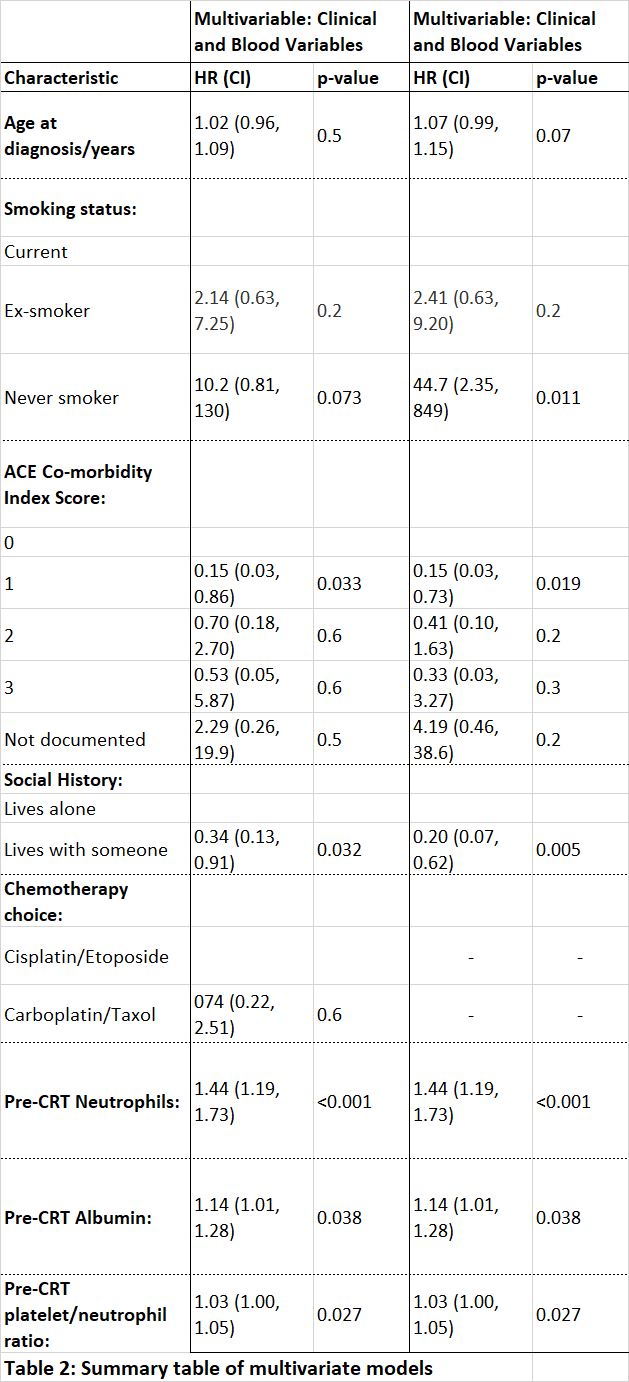
20/53 collected variables were selected in bootstrapping. The AIC for the model including both clinical and blood selected variables (AIC=174) was significantly lower (p≤0.001) than that using clinical variables alone (AIC=192), indicating improved model performance when blood information is included (see table 2).
Conclusion
Our multivariable models show that there is prognostic information within routinely collected blood and clinical variables in patients undergoing CRT and consolidation IO for locally advanced NSCLC. Expansion of the cohort and further validation in similar cohorts are required to investigate if these variables remain significant and therefore have clinical utility.