Response to induction chemotherapy on PET/CT predicts post-RT progression in patients with NSCLC
PO-1306
Abstract
Response to induction chemotherapy on PET/CT predicts post-RT progression in patients with NSCLC
Authors: Marie Tvilum1, Marianne Marquard Knap2, Azza A. Khalil3, Lone Hoffmann4, Christina M. Lutz4, Ane Appelt5, Hjørdis H. Schmidt3, Ditte Sloth Møller4
1Aarhus University Hospital, Danish Center for Particle Therapy, Aarhus, Denmark; 2Aarhus University Hospital, Dept. of Oncology, Aarhus , Denmark; 3Aarhus University Hospital, Dept. of Oncology, Aarhus, Denmark; 4Aarhus University Hospital, Dept. of Medical Physics, Aarhus, Denmark; 5University of Leeds, Institute of Medical Research at St. James's, Leeds, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Patients with locally advanced (LA) non-small cell lung cancer (NSCLC) are treated with curatively intended combined chemo-radiotherapy (cCRT). However, overall- and progression-free survival remain poor. Selecting patients for treatment intensification could be the key to improve outcome for this group of patients. To date, imaging features are only rarely used for treatment intensification, as reliable correlations have yet to be established. This study investigates whether radiologic and metabolic response to induction chemotherapy can predict freedom from progression in patients with LA-NSCLC treated with cCRT.
Material and Methods
Patients with LA NSCLC treated with cCRT at a single institution were included for analysis (n=199). Baseline diagnostic (dPCT) PET-CT and planning (pPCT) PET/CT-scans were collected. Patients received induction platinum-based chemotherapy between the two scans. Inclusion criteria for this study were treatment with ≥60 Gy RT dose, and no surgical intervention. Gross tumour volume (GTV-T) was delineated at pPCT, deformably transferred to dPCT, and corrected if needed. Changes in volume and SUVpeak for GTV-T were calculated. Patients were divided into subgroups based on their relative volume- and SUVpeak-response (below/above median). Event was defined as progression at any site. Freedom from progression was calculated from the date of the pPCT and analyzed using the Kaplan Meier method. Cox proportional hazards model for progression was performed including performance status, stage, histology, gender, log of the baseline GTV-T volume, SUVpeak at baseline, and relative changes in volume- and SUVpeak -values from pre- to post-chemotherapy.
Results
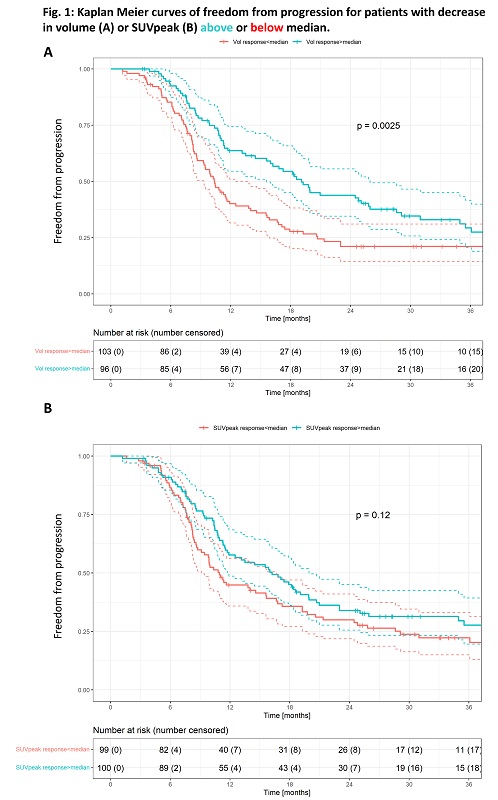
Median follow-up time was 43 months. Baseline patient characteristics are shown in Table 1. Median change in volume was a decrease of 18% (IQR: -9.9%;-27.0%). Median change in SUVpeak was a decrease of 33.6% (IQR: -19.6%;-47.9%). Kaplan Meier curves for patients split by relative decrease in volume and SUVpeak (below/above median) are shown in figure 1. Two-year freedom from progression was 43.8% (95%-CI: [0.35-0.56]) for volume responders above median, and 21% (95%-CI: [0.14-0.31]) for volume responders below median. No significant correlation between freedom from progression and decrease in SUVpeak was observed (p=0.12). The Cox proportional hazards model showed poorer freedom from progression for patients with adenocarcinomas (HR: 1.52 [1.00-2.2.31], p=0.049), a significant impact of baseline GTV-T volume (HR: 1.26 [1.09-1.46], p<0.01) and improved outcome for patients with the most GTV-T volume reduction (above median) (HR: 0.53 [0.37-0.76], p<0.01).
Conclusion
Patients with a large decrease in tumour volume after induction chemotherapy for locally advanced NSCLC have significantly better freedom from progression after treatment with cCRT. Patients with minor radiologic response to induction chemotherapy might benefit from treatment intensification.