Cardiological monitoring and safety of 5-fraction whole breast irradiation: SAFE-FORWARD study
Carlotta Becherini,
Italy
PO-1269
Abstract
Cardiological monitoring and safety of 5-fraction whole breast irradiation: SAFE-FORWARD study
Authors: Carlotta Becherini1, Chiara Bellini1, Giuseppe Barletta2, Luca Visani3, Livia Marrazzo4, Erika Scoccimarro1, Cecilia Cerbai1, Giulio Frosini1, Anna Peruzzi1, Ilaria Bonaparte1, Beatrice Bettazzi1, Victoria Lorenzetti1, Viola Salvestrini3, Marianna Valzano1, Monica Mangoni1, Marta Casati4, Stefania Pallotta4, Icro Meattini1, Lorenzo Livi1
1AOU Careggi - University of Florence, Radiotherapy Unit, Florence, Italy; 2AOU Careggi - University of Florence, Diagnostic Cardiology, Cardiothoracic, and Vascular Department, Florence, Italy; 3Istituto Fiorentino di Cura e Assistenza (IFCA), CyberKnife Center, Florence, Italy; 4AOU Careggi - University of Florence, Medical Physics Unit, Oncology Department, Florence, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
This study aims to assess heart toxic effect using a reliable cardiac assessment with standard and 3-dimensional (3D) echocardiography and left ventricular (LV) global longitudinal strain (GLS) in patients receiving a 5-fraction (total dose 26Gy) postoperative radiation therapy (RT) for breast cancer (BC).
Material and Methods
SAFE-FORWARD is an observational prospective cohort study (NCT04842409). Patient population included both invasive and ductal carcinoma in situ (DCIS) BC receiving ultra-hypofractionated whole breast irradiation (WBI) after breast conserving surgery (BCS). All enrolled patients are prospectively monitored for 12 months, receiving a complex cardiological assessment before RT start (baseline), and at 2-, 6-, and 12-month after RT end of treatment. Both acute and early-late toxicity are scored according to EORTC/RTOG and CTCAE (v.5) scales. The primary endpoint is defined as detection of any subclinical impairment in myocardial function and deformation (decrease ≥10%) measured with standard and 3D echocardiography and LV GLS.
Results
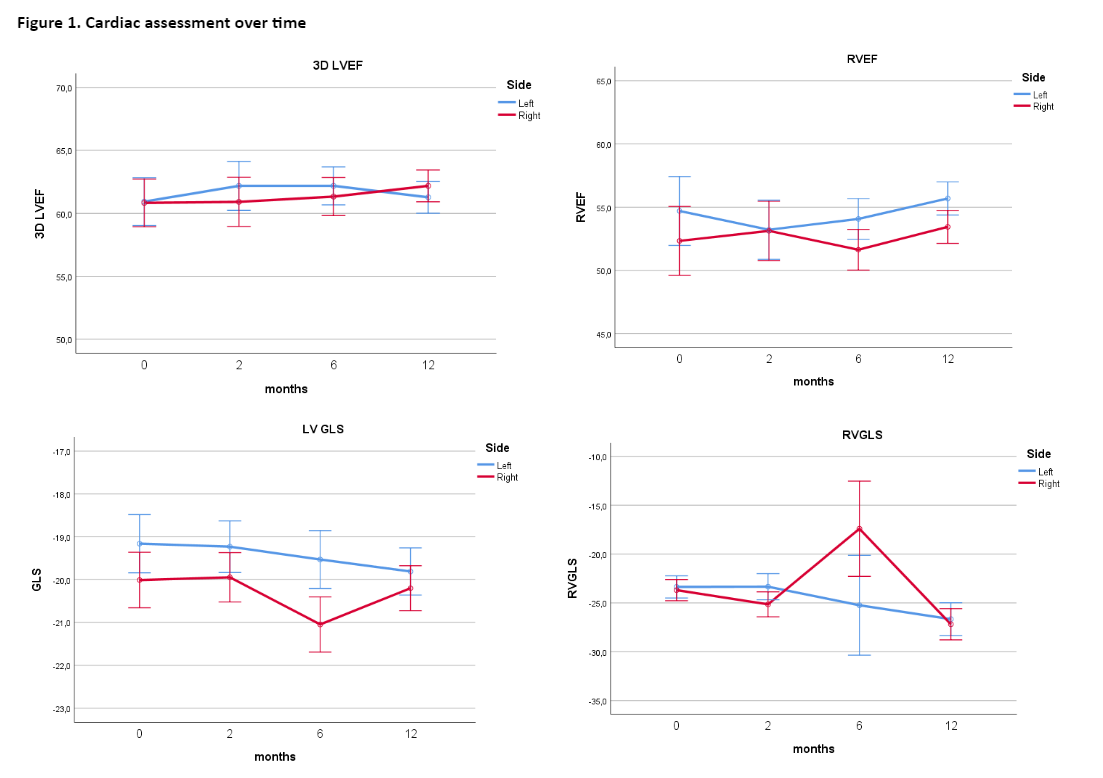
Overall, 40 women (median age 66 years; range, 48-84) were enrolled in the study. We analysed patients who had completed the cardiological assessment at 12 months. Baseline demographic, tumour, and cardiovascular profiles are summarised in Table 1. All patients received ultra-hypofractionated WBI (26 Gy in 5 fractions), 25 patients also received adjuvant endocrine therapy.
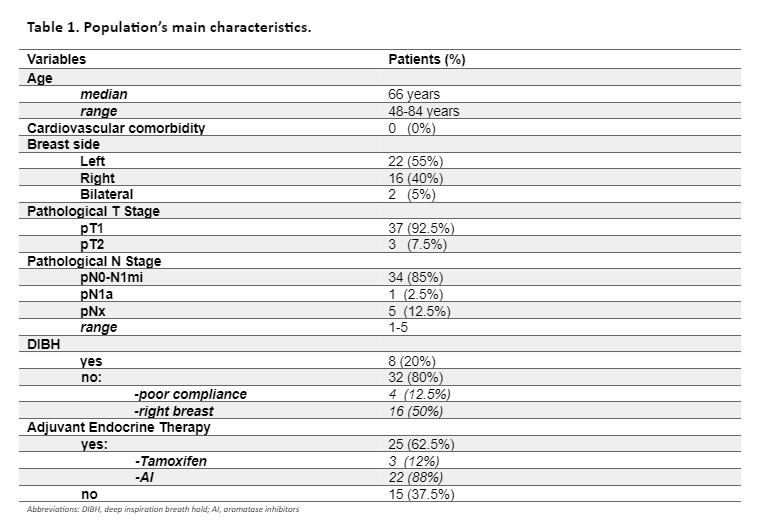
GLS worsened 4% or less, both for the left- and right-side treated breast, and remained in normal range for all the time points. The only exception was for RVGLS at 6 months for right-sided treatment where it reached a borderline value (-17.4±4.9 SE). 3D-LVEF remains stable during observation, both for the left- and right-side treated breast (Figure 1).
Conclusion
5-fraction schedule after BCS is well tolerated and the intensive 1-year cardiological monitoring showed no significant differences overtime in cardiac functioning.