Compliance analysis in a phase III study of esophagus-sparring palliative spine irradiation.
Anna Mann Nielsen,
Denmark
PO-1086
Abstract
Compliance analysis in a phase III study of esophagus-sparring palliative spine irradiation.
Authors: Anna Mann Nielsen1, Katrine Smedegaard Storm2, Morten Hiul Suppli3, Ann Christin Lund3, Claus Behrens1, Patrik Sibolt1, Helle Pappot3, Ivan Richter Vogelius3, Gitte Persson1
1Copenhagen University Hospital – Herlev and Gentofte, Department of Oncology, Copenhagen, Denmark; 2Copenhagen University Hospital – Herlev and Gentofte, Department of Oncology, Copenhagen, Denmark; 3Copenhagen University Hospital – Rigshospitalet, Department of Oncology, Copenhagen, Denmark
Show Affiliations
Hide Affiliations
Purpose or Objective
The ESO-SPARE trial investigates if esophagus sparring radiotherapy (RT) can reduce patient reported esophagitis in patients with cervical and thoracic metastatic spinal cord compression (MSCC). As the patient cohort is fragile, a considerable drop out was expected. An interim analysis of patient’s adherence to protocol and drop-out rate was planned as a quality assurance of the sample estimation.
Material and Methods
In ESO-SPARE (ClinicalTrials.gov Identifier: NCT05109819) patients are randomized to standard or esophagus sparing volumetric modulated radiotherapy in any fractionation. In the experimental arm, esophagus dose may not to exceed a certain biological equivalent dose (EQD2 < 8 Gy, α/β=3) and this is prioritized higher than CTV and PTV coverage.
Follow-up (FU) consists exclusively of Patient Reported Outcomes (PROs). The symptoms dysphagia, nausea, dyspepsia, and pain are reported daily for 5 weeks and weekly for additional 4 weeks by PRO-CTCAE. Use of analgesics, weight, and quality of life (EQD-5 and EORTC QLQ C30 questionnaires) are reported weekly for 9 weeks. Patients are included by radiotherapy technologists or physicians. Only patients deemed fit to complete 9 weeks FU are included in the study.
Co-primary endpoints are 1) severity of dysphagia, reported as the maximum PRO-CTC-AE score in the 5 weeks after treatment start and 2) ambulatory function at 9 weeks, assessed from EQ-5D questionnaires. A total of 200 patients from two centers are planned. To meet power calculations 62 patients in each arm are needed.
The interim analysis was planned after inclusion of 50 patients. Patients who did not answer any questionnaires after baseline were categorized as non- responders, the rest as responders. We assessed how many patients completed 5- and 9-weeks follow-up (timepoints of primary endpoint analysis).
Results
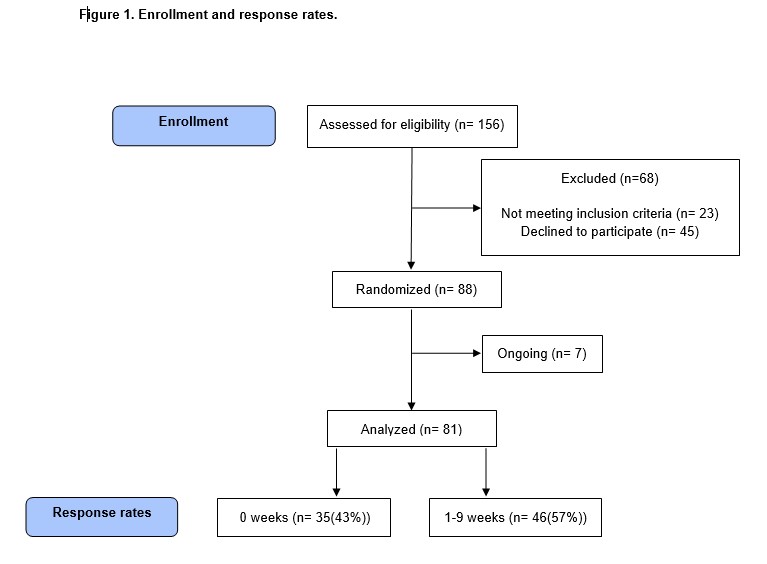
The interim was carried out in September 2022 after inclusion of 88 patients. 81 patients were included in the analysis, Figure 1 and Table 1.
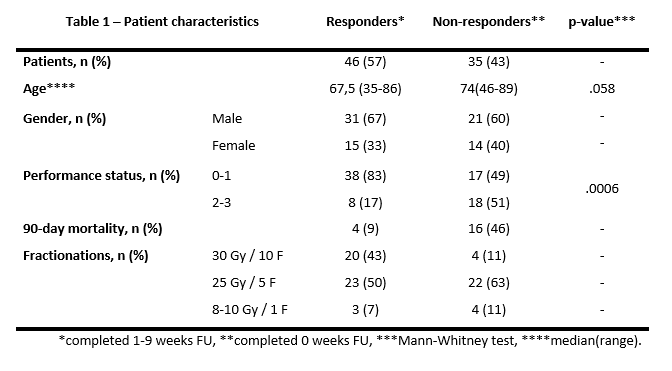
A total of 39 (52%) patients completed 5 weeks follow-up and 33 patients (40%) completed 9 weeks of follow-up. As many as 35 patients (43%) were non-responders. Non-responders had a significantly worse performance status (p=0.0006) and 90-day mortality was 46% compared to 9% in responders.
Conclusion
With the current compliance rate, the total number of evaluable patients will be too low, and the study under-powered. The low response rate can be explained by the fragile patient population with rapid decline in performance and short overall survival.
To increase compliance, we have introduced electronical SMS-questionnaires, weekly reminders by telephone and a possibility of telephone guidance 2-3 times weekly. If this does not increase compliance considerably, an increased number of participants is needed.