Development of a patient decision aid prototype for treatment of muscle invasive bladder cancer
Samantha Sigurdson,
Canada
PO-1093
Abstract
Development of a patient decision aid prototype for treatment of muscle invasive bladder cancer
Authors: Samantha Sigurdson1, Himu Lukka1
1McMaster University, Oncology, Hamilton, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
Patients with muscle invasive bladder cancer may be candidates for bladder preservation with trimodality therapy (TMT) consisting of maximal tumor resection and concurrent chemoradiotherapy, or radical cystectomy (RC) with ileal conduit. This choice is life altering due to the significant differences in modality and quality of life associated with each treatment option. It is difficult for patients to understand the major decision they are facing and the associated harms, benefits, and functional outcomes during the few clinical encounters they will have with their oncologists. A patient decision aid (PtDA) is a clinical tool that promotes shared decision making by providing information about management options and helping patients understand which values are most important to them. We developed a prototype PtDA for individuals who are offered TMT or RC with ileal conduit to improve their knowledge of options and outcomes.
Material and Methods
We used the International Patient Decision Aids Standards (IPDAS) to guide the systematic development process and ensure minimal risk of bias. A literature review was performed to determine the options and incidence of outcomes.
Results
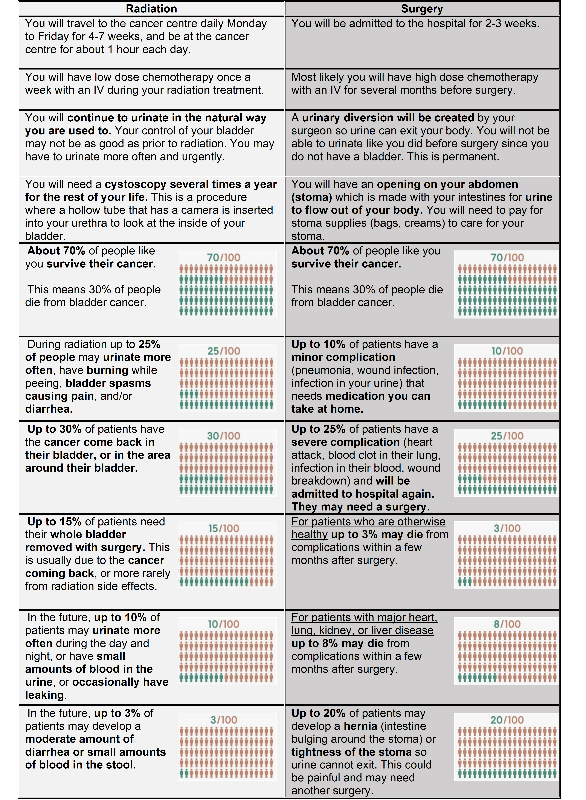
Ileal conduit was included as the primary urinary diversion option since this is the diversion most commonly performed at our centre, especially for patients who are also candidates for TMT as these patients are generally older or have higher burden of comorbidities than patients who are candidates for a continent neobladder. The PtDA highlights the difference in process between TMT and RC with ileal conduit, specifically evidence for each option, time in the hospital or cancer centre, and urination method after treatment. Outcomes specific to ileal conduit were post-operative morbidity and mortality, stomal stenosis, and parastomal hernia. Outcomes specific to TMT were acute and late toxicity, and salvage cystectomy. The PtDA emphas the similar disease-free survival expected with either treatment option.
Conclusion
PtDAs improve patient knowledge of the health condition, options, and outcomes. We created a novel PtDA prototype to improve the quality of decisions made by patients when deciding between TMT and RC with ileal conduit. Acceptability and effectiveness testing will be performed prospectively with uro-oncologists, medical and radiation oncologists, and patients.
Figure 1: PtDA for bladder cancer patients to make an informed choice between surgery and radiation.