IMPT reduces esophageal and pulmonary toxicity compared to VMAT in stage II-IV NSCLC patients
Arno Hessels,
The Netherlands
MO-0470
Abstract
IMPT reduces esophageal and pulmonary toxicity compared to VMAT in stage II-IV NSCLC patients
Authors: Arno Hessels1, Rutger Stoffers1, Anne Niezink1, Olga Chouvalova1, Fred Ubbels1, Annija van der Leest1, Marleen Woltman - van Iersel1, Pieter Deseyne1, Harriët Elzinga1, Ellen Haan - Stijntjes1, Erik Korevaar1, Pietro Pisciotta1, Hans Langendijk1, Robin Wijsman1
1University Medical Center Groningen, Radiation Oncology, Groningen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
The objective of this study was to evaluate whether observed rates of grade≥2 radiation pneumonitis (RP) and grade≥2 acute esophagitis (AE) in NSCLC patients treated with chemoradiotherapy using either IMPT or VMAT matched predicted rates of toxicity according to the Dutch National Indication Protocol Proton Therapy for Thoracic tumors that is used to select patients for IMPT.
Material and Methods
Patients were selected for IMPT or VMAT based on expected reduction of normal tissue complication probabilities (ΔNTCP) for grade≥2 RP, grade≥2 AE, and/or 2-year mortality based on a treatment plan comparison (VMAT vs IMPT). Toxicity rates of RP and AE were prospectively collected as part of our standard follow-up program (SFP) for lung cancer patients (Clinicaltrials.gov: NCT02421718). To assess the benefit of IMPT over VMAT, model-based clinical evaluation was applied, comparing the observed toxicity rates after IMPT to the expected rates calculated from the VMAT plans made for plan comparison for each patient treated with IMPT. Additionally, observed RP and AE rates were compared with expected rates of the same technique to validate the NTCP-models. Multivariable logistic regression analysis was performed to adjust for potential confounders (WHO performance score, age, smoking status, tumor stage, immunotherapy use and mean radiation doses for heart, lungs and esophagus).
Results
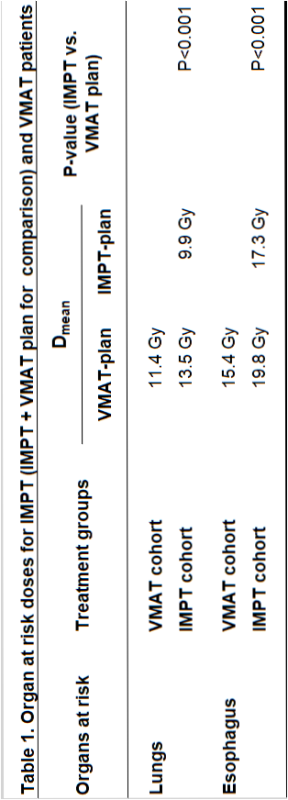
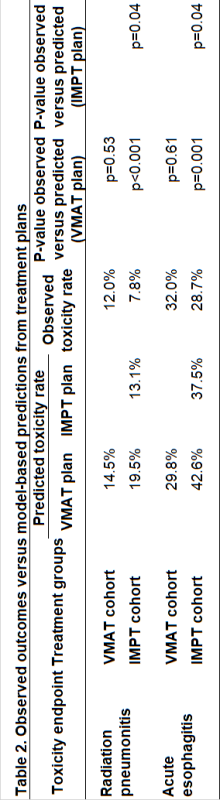
214 patients were treated between 10-2019 and 03-2022, 108 (50%) receiving IMPT, 106 (50%) receiving VMAT and 135 (63%) receiving adjuvant immunotherapy. With IMPT, mean lung dose and mean esophagus dose were significantly lower compared to VMAT (Table 1). In VMAT patients, observed rates of RP and AE corresponded well with predictions (mean NTCP) indicating that the model can be used without adjustment for the model-based clinical evaluation (Table 2). The observed rates of RP and AE after IMPT were significantly lower than predicted both from their VMAT plans made for plan comparison (RP: OR 0.35, 95% CI 0.11-0.61; AE: OR 0.54, 95% CI 0.34-0.81) and IMPT plans (Table 2). After adjusting for confounders, IMPT patients had lower observed RP (OR 0.28, 95% CI 0.09-0.92; p=0.04) and AE rates (OR 0.37, 95% CI 0.17-0.80; p=0.01) compared to observed rates of VMAT patients. The observed RP and AE rates after IMPT were significantly lower than expected based on the NTCP-model, indicating model adjustment is needed for IMPT plans.
Conclusion
The observed rates of RP and AE for patients treated with IMPT were significantly lower compared to the predicted rates from their VMAT-plans made for plan comparison and, based on multivariable analysis, compared to the observed rates of VMAT patients. The photon-based NTCP-models for RP and AE predict the NTCP for VMAT accurately but overestimated the risk of RP and AE for IMPT plans.