The addition of immunotherapy to lung cancer chemoradiotherapy: Factors associated with survival
MO-0469
Abstract
The addition of immunotherapy to lung cancer chemoradiotherapy: Factors associated with survival
Authors: Huei-Tyng Huang1, Douglas H. Brand1, John D. Fenwick2, Maria A. Hawkins1
1University College London, Medical Physics and Biomedical Engineering, London, United Kingdom; 2University of Liverpool, Department of Molecular and Clinical Cancer Medicine, Liverpool, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
For inoperable locally advanced non-small cell lung cancer (LA-NSCLC), treatment outcomes have been improved by adding immune checkpoint blockade (ICB) to standard concurrent chemoradiotherapy (cCRT). Trials of cCRT-ICB are heterogenous for factors such as tumour histology, PDL1 status and radiotherapy schedules. We aimed to model the ICB contribution to survival across published trials and determine whether ICB regimen-specific factors were associated with survival.
Material and Methods
Observed rates of 2-year overall survival (OS) were retrieved from published cCRT-ICB studies. OS rates for cCRT alone were estimated using a published dose-response model fitted to 2-year OS rates for treatments delivering chemoradiotherapy without ICB. Net contributions (‘OS gain’) of the various ICB regimens were quantified by subtracting these model estimates of OS by cCRT alone from the published OS rates for cCRT+ICB. A meta-regression approach was used to analyse whether OS (and hence OS gain) were associated with ICB, patient and clinical factors. Survival models were fitted using maximum-likelihood estimation, comparing fit quality via the Akaike Information Criteria and likelihood ratio test.
Results
Population data were collated from prospective trials and institutional series with >20 cases published between 2018-2022 (Table 1). 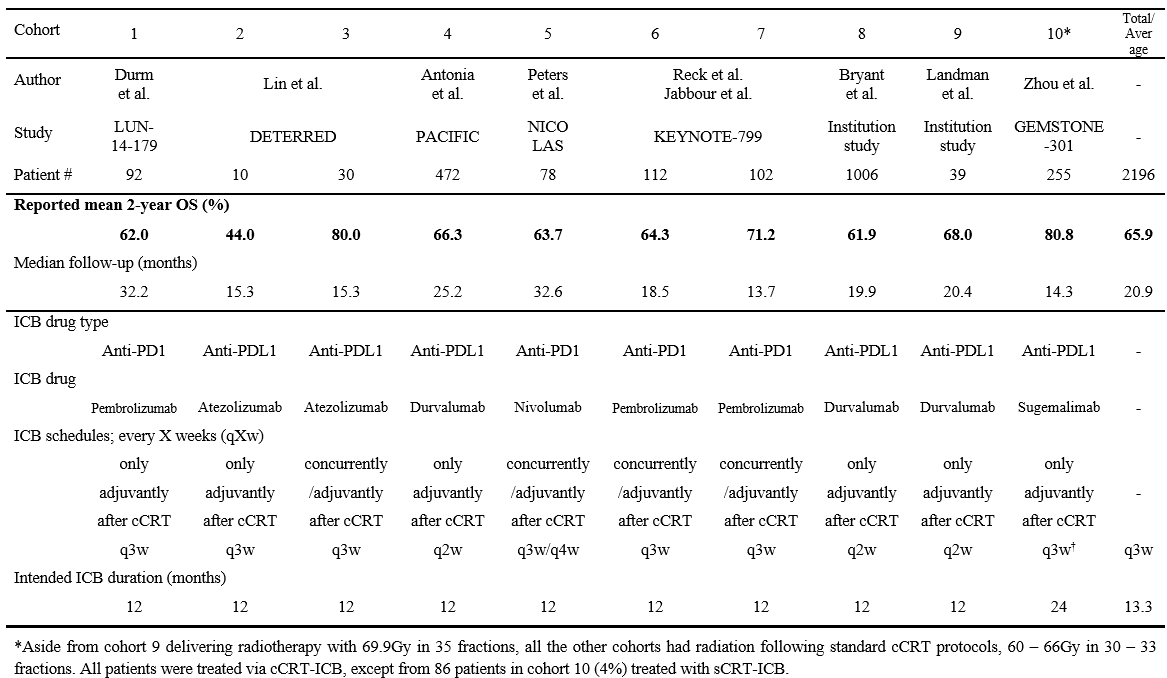 The data comprised 2196 NSCLC patients (99% stage III) treated in 10 cohorts using different receiving cCRT-ICB regimens. Planned RT schedules were 60-66Gy in 30-33 fractions for 9 cohorts and 69.9Gy in 35 fractions for one cohort. Six and four cohorts respectively received anti-PDL1 and anti-PD1 ICBs. ICB was given concurrently/adjuvantly for 4 cohorts and adjuvantly for 6 cohorts. Intended ICB duration was 24 months for one cohort and 12 months for 9 cohorts.
The data comprised 2196 NSCLC patients (99% stage III) treated in 10 cohorts using different receiving cCRT-ICB regimens. Planned RT schedules were 60-66Gy in 30-33 fractions for 9 cohorts and 69.9Gy in 35 fractions for one cohort. Six and four cohorts respectively received anti-PDL1 and anti-PD1 ICBs. ICB was given concurrently/adjuvantly for 4 cohorts and adjuvantly for 6 cohorts. Intended ICB duration was 24 months for one cohort and 12 months for 9 cohorts.
In the meta-regression (Table 2), ICB significantly improved survival relative to cCRT alone, producing an estimated 2-year OS gain of 8.6% (95% CI: 5.3-12.2%, p<0.001). However, ICB drug-type (anti-PDL1 vs anti-PD1), ICB timing (concurrent/adjuvant vs adjuvant alone), RT dose and RT duration were not associated with the OS gains achieved using different ICB regimens. In terms of 2-year OS, intended ICB duration was positively associated with OS (p=0.011), while the fraction of tumour PDL1% >1% (p=0.009) and median age (p<0.001) were negatively associated with OS.
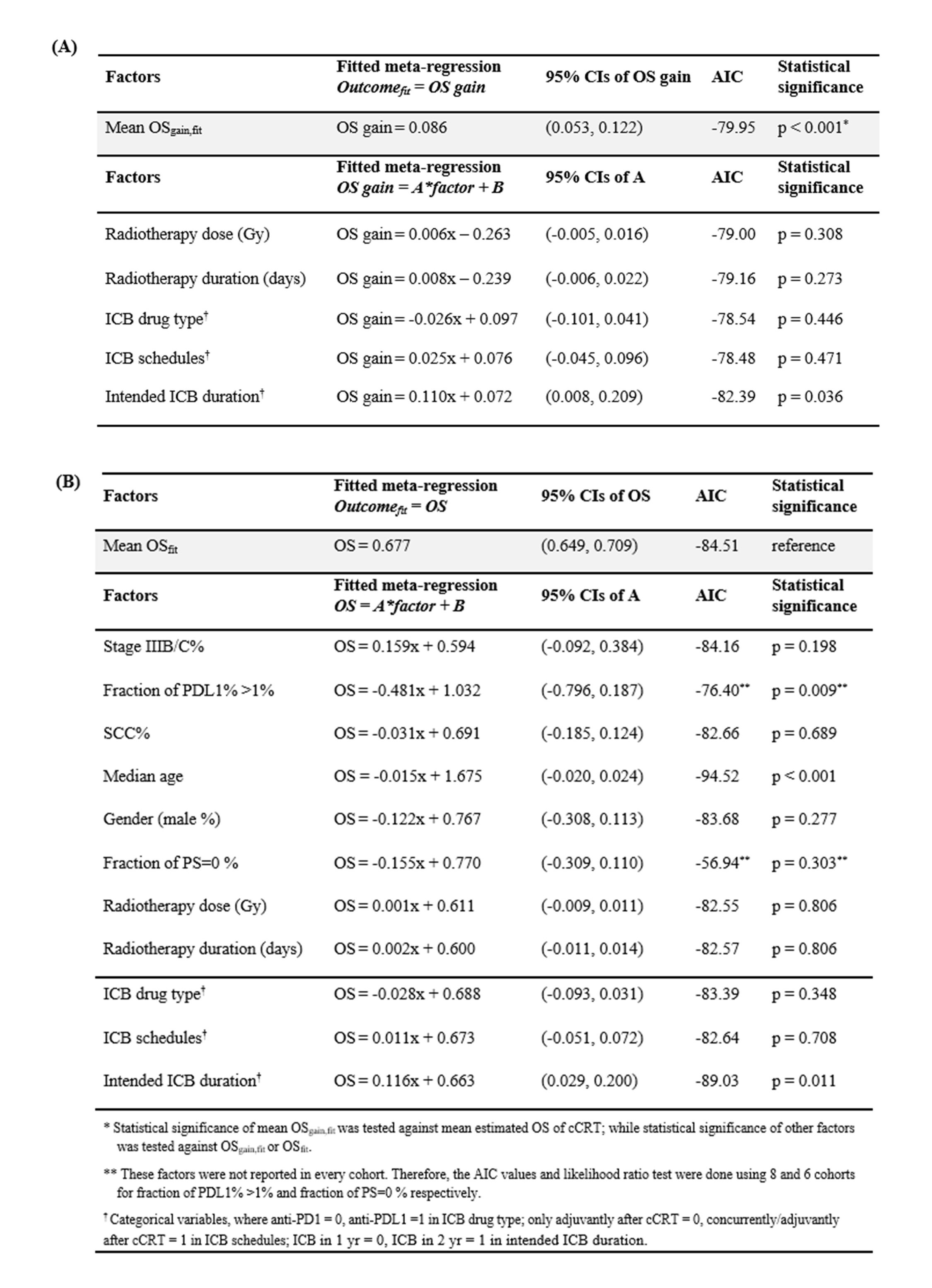
Conclusion
The quantified ICB contribution corroborates the PACIFIC trial finding that ICB provides significant OS improvement following standard cCRT regardless of disease stage and histology, with 8.6% (95% CI: 5.3-12.2%) 2-year OS gain. Intended ICB duration, fraction of tumour PDL1%, and age were significantly associated with the treatment outcomes. Tumour biology and molecular features need to be studied to facilitate patient selection as the benefits from cCRT-ICB may be greater for specific subgroups.