Modulated electro-hyperthermia improves three year survival in cervical cancer patients
Carrie Minnaar ,
South Africa
PH-0551
Abstract
Modulated electro-hyperthermia improves three year survival in cervical cancer patients
Authors: Carrie Minnaar 1, Jeffrey Kotzen2, Ans Baeyens3, Mboyo-Di-Tamba Vangu1, Carrie Minnaar2
1University of the Witwatersrand, Radiation Sciences, Johannesburg, South Africa; 2Wits Donald Gordon Medical Centre, Radiation Oncology, Johannesburg, South Africa; 3Ghent University, Department of Human Structure and Repair, Ghent, Belgium
Show Affiliations
Hide Affiliations
Purpose or Objective
OBJECTIVE:
Modulated electro-hyperthermia (mEHT) is a capacitive coupled hyperthermia technique
which transmits amplitude modulated 13.56MHz radiofrequency waves between two
electrodes. mEHT is being investigated as an adjunct to chemoradiotherapy (CRT)
in a Phase III Randomised Controlled Trial
for locally advanced cervical cancer patients in South Africa. We reported
previously on the improved local disease control with the addition of mEHT (45.5% versus 24.1%; p=0.003), without any significant effect
on early toxicity, and a potential abscopal response in participants in whom
extra-pelvic nodal disease was visualized on the pre-treatment 18F-FDG
PET/CT studies. In this report we present updated data on three
year survival.
Material and Methods
MATERIALS
AND METHODS: Inclusion criteria: FIGO stages IIB to IIIB carcinoma
of the cervix, eligible for CRT with radical intent. HIV-positive participants were
included provided they had a CD4 count >200cells/µL or had been treated with
antiretroviral therapy for >6 months. All participants were required to sign
an informed consent. 210 Participants were randomized into a control group and an
intervention (mEHT) group (stratum: HIV status, accounting for age and stage).
Both groups received CRT: 50Gy/25fractions external beam radiation (EBRT); three
fractions of 8Gy HDR Brachytherapy; and two doses of cisplatin (80mg/m2). The mEHT
group received two mEHT treatments per week (55 minutes at 130W), immediately
before EBRT. Disease response was assessed at six months post treatment. Approval
was obtained from the local ethics committee (M190295) and the trial was
registered on the National Clinical Trials Register (ID:3012) prior to the enrolment
of participants, (ClincialTrials.gov ID: NCT03332069).
Results
98 out
of 106[93%] and 99 out of 104[95%] participants in the mEHT and control groups respectively,
were evaluated at three years. Participants in the mEHT group were
significantly more likely to achieve three year all mortality survival (n=48[49%])
than those in the control group (n=38[38%]) (HR: 1.45;
95%CI: 1.0-2.1; p=0.044). Disease
free survival at three years was significantly more likely in the mEHT group
(n=33[34%]) than in the control group (n=14[14%]; p=0.001), (OR:2.4; 95%CI: 1.3-4.4; p=0.003). In the subset of participants in which a potential abscopal
response was observed, 10 of the 13 participants in the mEHT group, and 2 of
the 3 in the control group, are alive and disease free at three. There were no
significant differences in late toxicities between the groups.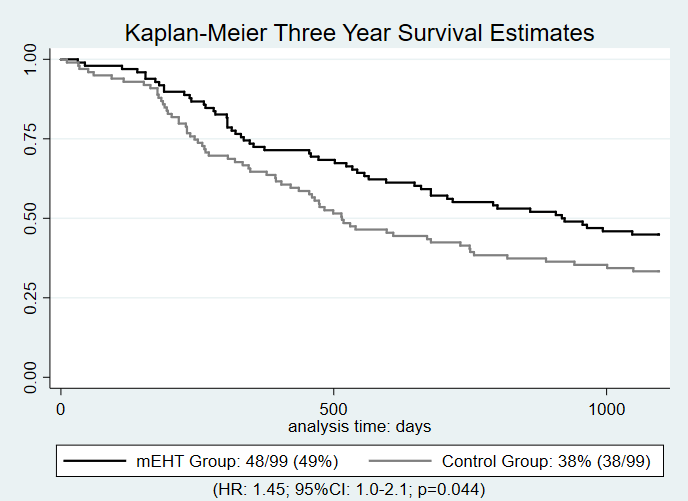
Conclusion
CONCLUSION:
mEHT improves local disease control as well as three year survival and disease
free survival at three years. These results provide strong motivation for the
incorporation of mEHT into clinical practices as a radiosensitiser for locally
advanced cervical cancer, especially in resource constrained settings.