Targeting Serine/Glycine Metabolism with the Repurposed Drug Sertraline: Impact on Cancer Cells and the Tumour Microenvironment
ESTRO 2024 Congress report
Metabolic alterations in tumour cells are recognised as important hallmarks of tumourigenesis (1). Among these, the hyperactivation of the de novo serine/glycine synthesis metabolic pathway has been observed across many different cancer types, strongly associated with tumour progression and resistance to conventional cancer therapies. For instance, patients with non-small-cell lung cancer (NSCLC) who express high amounts of serine/glycine synthesis enzymes, approximately 37% of cases, present lower overall survival rates compared with those cases that express low amounts of serine/glycine synthesis enzymes (Figure 1). Moreover, the limited activity of the de novo serine/glycine synthesis pathway in normal cells, which rely on the uptake of these amino acids from their microenvironment, makes serine/glycine synthesis hyperactivation a specific vulnerability of cancer cells and therefore an attractive therapeutic target for cancer treatment (2, 3).
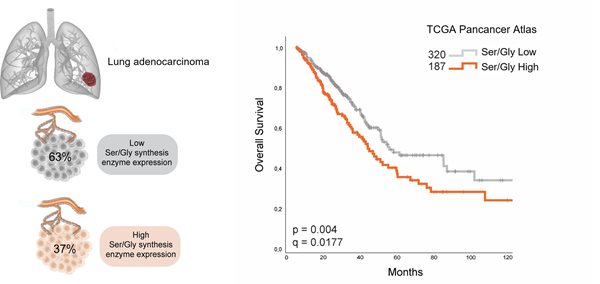
Figure 1. Strong expression of serine/glycine (Ser/Gly) enzymes in lung cancer is linked to poor outcomes. TCGA, The Cancer Genome Atlas dataset of lung adenocarcinoma (LUAD) patients. Median survival of LUAD that produces ser/gly (n=187) is 41.59 months (32.71 - 53.65) versus 53.33 months (47.80 - 88.14) in the ser/gly consuming group (n=320). Adapted from (4).
Our study sought to enhance our understanding of how serine/glycine synthesis is metabolically reprogrammed upon exposure to radiotherapy, as well as to explore the therapeutic implications of inhibition of serine/glycine metabolism through the use of the repurposed drug sertraline in NSCLC and glioblastoma (GBM). These represent cancers with high mortality rates and minimal therapeutic advancements over the last decades. A metabolomic analysis revealed that NSCLC cancer cells required serine and glycine metabolites under the selective pressure of radiotherapy. We observed increased expression of serine hydroxymethyltransferase-2 (SHMT2), the enzyme responsible for the interconversion of serine to glycine, both in vitro and in irradiated tumours in vivo (Figure 2A). The inhibition of serine/glycine synthesis through the use of sertraline, which specifically targets SHMT, in combination with radiotherapy was found to inhibit tumour growth and to sensitise tumour cells to the cytotoxic effects of radiotherapy, both in vitro and in vivo (Figure 2B). These findings highlight the survival benefit of serine and glycine metabolites for NSCLC cells after radiotherapy exposure, and they offer promising avenues by which to enhance radiotherapy responses in NSCLC.
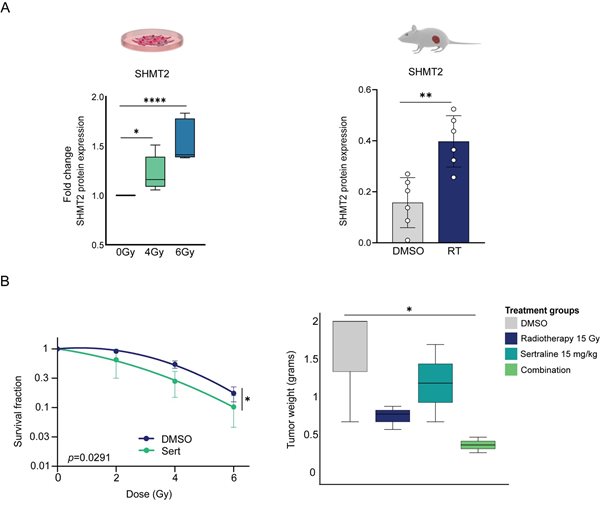
Figure 2. Inhibition of serine/glycine synthesis through the use of sertraline in combination with radiotherapy impairs the growth and survival of lung cancer cells. A) Left graph: fold change of SHMT2 protein expression in NSCLC cell lines in response to irradiation. Right graph: SHMT2 protein expression in lung tumours upon irradiation in vivo. An unpaired, two-tailed t-test was used to compare DMSO-treated mice and irradiated tumours. B) Left graph: survival curves for NSCLC cells as irradiation doses are increased from 2Gy to 4Gy and 6Gy, with and without sertraline. Survival fractions were determined relative to the non-irradiated cells with and without sertraline. Data were fitted to a quadratic linear model. Right graph: box plot showing tumour growth as tumour weight in the different treatment groups. Kruskal–Wallis test with a Dunn’s multiple comparisons test was performed. Adapted from (4).
Moreover, our investigation revealed the impact of serine/glycine metabolism on the tumour microenvironment and anti-tumour immune response. The metabolic activity of tumour cells can impact the behaviour of immune cells due to competition for available nutrients and the accumulation of metabolic products in the tumour microenvironment. In immunocompetent mice models that bore subcutaneous lung tumours that were treated with the combination of sertraline and radiotherapy, we observed decreased expression levels of the immunosuppressive protein galectin-1, along with increased expression of granzyme B, a cytotoxic molecule that is released by natural killer (NK) cells to eliminate tumour cells, which suggest enhanced anti-tumour functions of NK cells (Figure 3).
Within the 25% of GBM that overexpress serine/glycine pathway enzymes, approximately 13% of cases harbour genetic amplifications and mutations in the gene that encodes the enzyme phosphoserine phosphatase (PSPH), which generates serine. These genetic alterations in GBM underscore an intrinsic dependency on serine/glycine metabolism. Patient-derived GBM cells with PSPH overexpression demonstrated increased utilisation of serine and glycine for the synthesis of nucleotides and enhanced migratory capabilities. Moreover, PSPH amplification facilitated the expression of galectin-1. Accordingly, high PSPH expression could predict poor immunotherapy responses in patients with GBM. Collectively, serine/glycine synthesis pathway alterations and hyperactivation promote a proliferative and immunosuppressive GBM tumour phenotype that is associated with poor immunotherapy responses and outcomes.
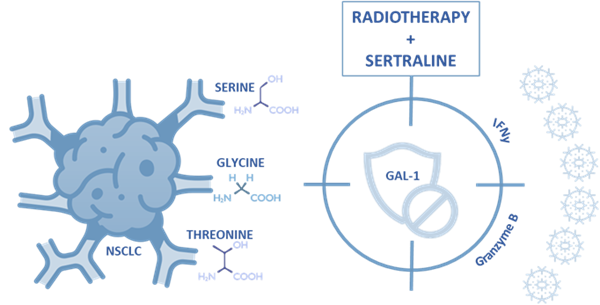
Figure 3. Serine/glycine pathway metabolic dependency in NSCLC can be inhibited by radiotherapy plus neoadjuvant sertraline treatment, simultaneously targeting galectin-1-mediated immunosuppression.
To exploit fully the therapeutic potential of inhibiting the serine/glycine synthesis pathway, crucial requisites are patient stratification and a better understanding of the interplay between serine/glycine synthesis dependency in tumour cells and immune cells within the tumour microenvironment. The use of this knowledge could increase the effectiveness and development of novel immunotherapies in cancer treatment. Our study challenges current therapeutic approaches and aims to enhance cancer patient outcomes through the use of (neo)adjuvant sertraline treatment, paving the way for future studies and clinical applications in cancer.

Anaís Sánchez Castillo, Department of Radiation Oncology (Maastro), GROW Research Institute for Oncology and Reproduction, Maastricht University Medical Centre+, Maastricht, The Netherlands.
e-mail: a.sanchezcastillo@maastrichtuniversity.nl
Website: https://www.maastrolab.com/