Evolution of the clinical routine on the CyberKnife® in Hungary - PDF Version
An Accuray CyberKnife® M6™ system was installed at the National Institute of Oncology, Budapest, Hungary, in 2018. It is a linear accelerator that is dedicated to the performance of stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT) treatments with real time intrafractional motion tracking (Image 1).
Image 1. Accuray CyberKnife® M6™ system at the National Institute of Oncology, Budapest, Hungary
Radiotherapists (RTTs) attended a one-week course to learn the handling of the system at the Accuray training centre, Sunnyvale, California. The company also provided us with a trainer for the first week of operation of the machine. The trainer helped us to manage the treatments during the so-called “go-live” week.
The radiation oncologist is present only for the first treatment; after this, RTTs treat the patients themselves. We have a senior physician who is dedicated to the CyberKnife on site, so in case of any problems, he’s there to help us. RTTs ask the patients about their condition before each repeat treatment, and if they report any side effects, the patients are directed to their radiation oncologists for further examination.
The RTTs have gained competence in the performance of daily dosimetric quality assurance tests and the contouring of the organs at risk (OAR) during the dedicated treatment-planning system. The department places the contouring of the OAR in the hands of a dedicated dosimetrist team; however, the CyberKnife treatments demand close collaboration between team members and it is beneficial for RTTs to gain experience in these areas.
Treatments begin through the use of three types of tracking methods:
6D skull and Xsight® spine algorithms both track the bone anatomy. The system acquires an orthogonal X-ray image pair at selected time intervals during treatment and detects the position of the irregularly shaped bones. 6D skull tracks the motion of the cranium, while XSight spine follows a predefined region of vertebrae.
The third method is fiducial tracking, which involves the use of implanted gold markers. The fiducials are inserted into patients at least 14 days before the planning computed tomography (CT), to avoid errors that may be caused by marker migration. The high density of these fiducials in soft tissues helps the system to track the movement of the selected organ (Image 2). During the first year of use, most of the patients for whom this tracking algorithm was used had low- and intermediate-risk prostate cancer and they received their treatment on every other working day.
.jpg.aspx)
Image 2. Fiducial tracking on the CyberKnife
We treated 294 patients in 2018. Most of them had brain tumours (165, malignant and benign), prostate cancer (76) and bone metastases (19). The figures for the last three years are shown in Figure 1.
.jpg.aspx)
Figure 1. Number of patients who were treated under the CyberKnife from 2018 to 2020, categorised according to bodily region in which the cancer occurred.
The Synchrony® and XSight Lung respiratory tracking systems were installed in late 2018. The staff received three days of onsite training, organised by Accuray, to learn this technique. The Synchrony compensates for the patient’s breathing motion by moving the treatment field during delivery, according to a correlation model. The respiratory motion of the chest wall is detected by an optical camera, which monitors three light-emitting diodes (LEDs) that are attached to the patient’s vest. The respiratory motion of the tumour is registered in 15 different phases of the breathing cycle on the X-ray images with fiducial tracking. These two sets of 3D information are correlated in the Synchrony model, in which the system can predict the tumour’s location according to the actual chest wall position (Image 3).
.jpg.aspx)
Image 3. Treating lung tumours through use of the CyberKnife with Synchrony and Xsight lung tracking algorithms
Through use of these methods, we treated our first lung-cancer patients (eight in 2018) and started to offer accelerated partial breast irradiation (APBI) (Figure 1).
For lung patients who were judged ineligible for marker implantation but eligible for CyberKnife SBRT treatment (T1-T2 primary, locally recurrent peripheral tumours, pulmonary metastases) we applied the XSight lung algorithm. The method is very similar to that used in Synchrony, but instead of fiducial detection the algorithm can recognise the projection of a lung tumour that is larger than 2cm.
In 2019, we incorporated APBI into our daily routine. We also treated hepatic metastases through use of Synchrony. We performed our first CyberKnife irradiations for children, whose treatment usually requires extra caution and sometimes anaesthesia. Our patient number increased dramatically, and hence it became necessary to extend working hours to two shifts. For the two shifts we increased our RTT number from three to five. The new team members learned the handling of the CyberKnife from the core staff and the physicists.
In 2019 we irradiated 728 patients through use of the CyberKnife. The most frequently treated tumour regions with standard tracking were brain (343), prostate (212) and bone (34), while with respiratory motion tracking these were breast (40) and lung (37) (Figure 1).
Last year brought the challenge of COVID, but we managed to keep the number of treated patients high. The top two treated regions with standard tracking were brain (340) and prostate (269), and with chest-wall motion tracking they were lung (29) and breast (14) (Figure 1).
We carried out our first re-irradiations of patients with prostate cancer who had previous seed implantation. In these cases, tracking required additional awareness because the seeds could overlie the fiducials on the image.
In October 2020, we received the Precision 2.0 upgrade with the new VOLO™ treatment planning optimiser, which was developed for a multi-leaf collimator (MLC). The lengths of treatment sessions that follow the VOLO-optimised plans are much shorter with similar plan quality (Figure 2). In comparison with the sequential optimised plans, the average reduction in door–to-door time is nine minutes, and this ranges from two minutes in the case of brain treatment, to 18 minutes in the case of prostate treatment.
.JPG.aspx)
Figure 2. Average door-to-door times measured for four different regions, with and without application of the VOLO algorithm
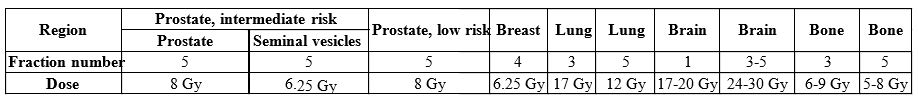
We can declare that, by the end of 2020, we could treat every generally known indication with application of the CyberKnife. On top of that, some special techniques such as APBI or those required for the treatment of children have become part of our routine. Data on fractionation for the most common regions are shown in Table 1. Acute side effects are rare and mild, according to our early results.1,2
Table 1. Fractionation data for the most common regions on the CyberKnife

Márton Vékás
Lead RTT
National Institute of Oncology
Budapest, Hungary
vekasmarton@hotmail.com