ESTRO 2024 congress report
Over the last 5 years, the rectal cancer journey has changed enormously with many more appropriate treatment options available depending on tumour stage, site, molecular markers as well as patient fitness and preference. During ESTRO the initial results of the Phase III GRECCAR 12 (NCT02514278) trial were presented, but most of the other Phase II/III trials affecting this change have been presented and published previously so it was invaluable to have a summary of these trials and hear what the speakers felt were the pertinent questions moving forward.
Dr Femke Peters began considering the options for those with early rectal cancer. She importantly highlighted that if patients are going for surgery, there is no role for neo-adjuvant radiotherapy. However, Dr Peters explained many patients will accept a reduced cure rate to avoid radical surgery and we now have a number of options to offer these patients offering anything from a 60% - 90% organ preservation rate. Figure 1 highlights the trials discussed.
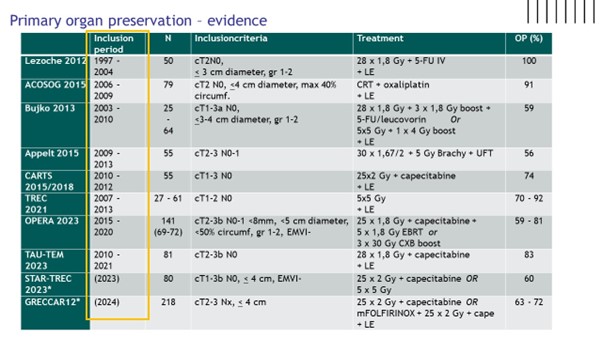
Dr Peters highlighted the PROMS available from TREC, CARTS, GRECCAR 2 and OPERA which overall were encouraging. However, particularly where local excision was used, as it was for selected patients in all trials, the rate of major bowel dysfunction was high. The GRECCAR 2 trial tried to address increased toxicity with a composite endpoint incorporating oncological endpoints as well as morbidity and side effects. They reported no difference between the TME and the organ preservation group using this composite endpoint and reported the rate of morbidity went up significantly as additional modalities were added. This highlights the point made by Dr Peters that these are a different group than the “accidental cCR”. These patients could have radical surgery alone so in the consent process we should be very clear that a failed attempt of organ preservation will leave them with worse QoL.
The trials to date have left several avenues for future investigation. Response prediction will facilitate improved patient selection and there are a lot of groups working on this. Wider use of contact or potentially brachytherapy, the use of an EBRT boost potentially with MRL, and the addition of chemo or immunotherapy as done in GRECCAR 12 trial presented earlier in the meeting are all strategies that require further thought. Or indeed the use of chemotherapy or immunotherapy alone.
Finally, it was addressed both in the presentation and in the follow-up questions that organ preservation is probably best performed by Centres with an established organ preservation practise. They are more likely to have appropriate trials up and running and have the required expertise in response assessment to pick up relapses at a timepoint that they are still salvageable. .
Professor Emmanouil Fokas reviewed the recent randomised Phase II/III trials in locally advanced rectal cancer, namely the Phase II CAO/ARO/AIO-12, Phase III’s RAPIDO and Prodige 23 and Phase II OPRA. The TNT versus RT then surgery trials of Prodige 23 and RAPIDO have confirmed there is improved DFS with TNT. As this is mostly due to the reduced distant metastasis-free survival, it is highly likely the chemotherapy prior to surgery is resulting in that impact. As a result, TNT should be considered in all patients. However, more work is required to address the impact on QoL, whether it remains appropriate in the elderly and frail and to identify biomarkers to avoid over-treatment. The trials addressing different TNT schedules all had identical DFS / OS in both arms. They are large enough to confirm that if the aim is a local response, as is the case in organ preservation, the order of treatments should be CRT then chemo as highlighted by OPRA and CAO/ARO/AIO-12. The number of patients required to confirm the optimal order of treatments to minimise distant metastasis and therefore optimise survival are prohibitively large for these trials.
The remaining questions are whether we should use FOLFIRINOX or FOLFOX within TNT, which will be addressed by the Phase III component of JANUS/NRG-GI010; and the role of contact in locally advanced disease, which will be addressed by the Phase III TRESOR looking at TNT using FOLFIRINOX with CRT with or without contact radiotherapy. Both the Phase III OPAXX study and CAO/ARO/AIO – 18.1 will address which RT regimen is optimal between SCRT and LCRT for organ preservation with OPAXX study use additional treatments within the protocol such as contact radiotherapy and local excision. All of the discussed planned trials have primary endpoints of organ preservation, which while relevant for some patients is vitally important we remember it will not be relevant to all.
Prof. Maria Antonietta Gambacorta tackled the challenging space of reirradiation in rectal cancer. There are a few things that have some evidence behind them in isolated locally recurrent rectal cancer. There is data that surgery with an R0 excision in patients who are fit for surgery offers the best long-term outcome for patients with isolated locally recurrent rectal cancer. We have consensus guidelines on the delineation of locally recurrent rectal cancer and suggested classifications of sites of recurrence. There is widespread acceptance we should be considering the cumulative dose to OAR’s in our planning calculations. However, due to the lack of prospective studies and the heterogeneous groups treated in all the mostly small, single-centre retrospective studies, there are significant uncertainties. Whether neo-adjuvant reirradiation can contribute to achieving an R0 resectionwas being asked by the GRECCAR 15 trial, which has unfortunately had to close early, results are awaited. PELVEX II trial delivers neo-adjuvant reirradiation to all patients randomising them to the addition of neo-adjuvant chemotherapy or not and will offer some unrandomised prospective evidence of reirradiation. The RETRY trial is looking at the use of carbon ion radiotherapy and TORCH-R is looking at combining systemic therapy with SABR in this setting. However, due to the heterogeneity of reirradiation aims and delivery, prospective trials are challenging therefore we should be submitting data to the ReCare (EORTC 2011-RP) database to maximise our learning from a practice that is set to increase rapidly over the next decade.
Finally, Professor Marta Scorsetti discussed the role of SABR in oligometastatic disease. The Guckenberg classification of oligometastatic disease was highlighted. This allows the aim of treatment to be considered, which may be curative or palliative. The aims can be improved local control to improve QoL, prolong PFS, prolong OS, prolong systemic therapy efficacy, delay the need for systemic therapy, and lastly enhance systemic therapy. There is limited prospective evidence looking specifically at colorectal cancer and as such it is important to look outside the radiation field at trials using different modalities to identify when there is a role for oligometastatic-directed therapy such as the Ruers et al CLOCC study using liver ablation in colorectal cancer, which demonstrated overall survival in metachronous oligorecurrence and metachronous oligoprogression. However, large-scale retrospective data can offer some predictors of improved OS; such as the site of treated metastasis (lung fair better than non-lung), larger volumes, previous systemic therapy, control of treated metastasis and a delivered BED >100Gy. Ultimately there are many pros of SABR as an oligometastatic-directed therapy and ongoing trials as well as large-scale databases such as OligoCare (EORTC 1822-RP) will no doubt further consolidate its use in this setting.
Rectal cancer is a constantly moving feat, so while “journey” is a word often disliked by patients to describe their individual experience, it accurately describes our experience of the constant motion of rectal cancer management. Year on year, the field continues to roll forward, through the fantastic collaborative work of healthcare professionals and patients around the world.

Rebecca Muirhead
Clinical Oncologist
Oxford University Hospital NHS Foundation Trust, UK.
Rebecca.muirhead@oncology.ox.ac.uk