ESTRO 2024 Congress report
During the last ESTRO congress, which was held in Glasgow, a primary endpoint analysis of the targeting resistant prostate cancer (TRAP) trial (NCT03644303) was presented. TRAP is a prospective phase-II trial in which the use of stereotactic body radiotherapy (SBRT) has been analysed in patients affected by oligoprogressive (metastatic) castrate-resistant prostate cancer (mCRPC). Briefly, 81 patients who progressed during treatment with androgen receptor pathway inhibitor (ARPi) and who had fewer than two oligoprogressive metastatic sites were treated with SBRT (either 30Gy in five fractions or 36Gy in six fractions for prostate) (Fig 1).
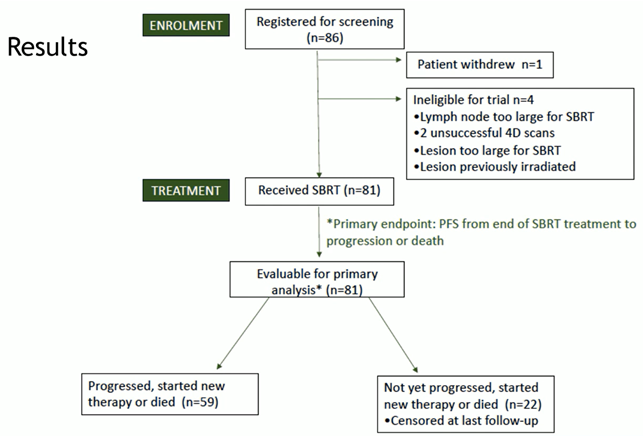
Fig1.
The trial results were positive, with a median progression-free survival (PFS) after SBRT of 6.4 months, which exceeded the expected four months in this scenario. Interestingly, SBRT provided a 40.1% PFS rate at 12 months, with a promising median time to the next treatment of 29.6 months. These data suggest that the use of SBRT may significantly prolong the clinical utility of ARPi. However, the authors of the study report underlined that 65% of patients progressed after a median follow-up of 19.2 months, with 40% of PFS events occurring within six months of SBRT (Fig 2).
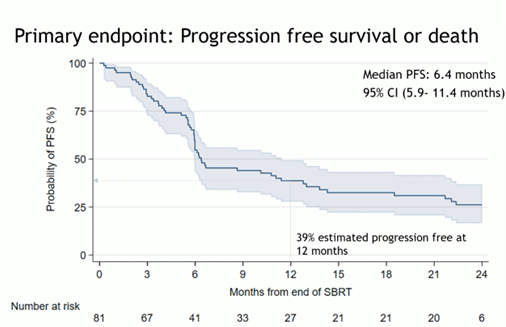
Fig 2.
These patients had a significantly different prognosis when compared with those who had been enrolled in the recently published ARTO trial (NCT03449719). This was a randomised phase-II trial that showed the significant benefit of the addition of SBRT to ARPi in terms of biochemical response and PFS in CRPC patients who were being treated with first-line abiraterone acetate. Indeed, a subgroup analysis of the ARTO trial that had been conducted on patients who were progressing after abiraterone treatment was presented at ESTRO 2024. The outcomes of SBRT that had been performed in oligoprogressive patients were presented, regardless of the original randomisation arm, compared with a second-line systemic treatment shift. The results suggested that, after a median follow-up of 14.8 months after the first progression event during abiraterone treatment, a second PFS event was detected in only 19% of the included patients. The reasons for these different prognostic outcomes are probably related to the differences between the populations included in the two trials (Fig 3).
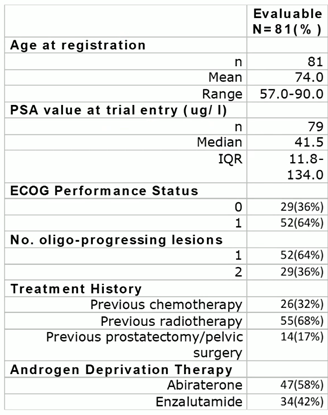
Fig 3.
First, the TRAP trial allowed the inclusion of patients with visceral metastases. This partly explains the worse prognosis reported after SBRT. However, only 1% of the included patients were affected by lung metastases, so further considerations are required.
Second, patients who were undergoing ARPi in the second or following lines of treatment were not excluded from the TRAP trial. In particular, 32% of the enrolled population had received a previous course of chemotherapy before the ARPi treatment. This underlines that these patients may have been a heavily pre-treated population compared with the first-line CRPC patients who were included in the ARTO trial. Moreover, patients were considered eligible for the TRAP trial if they were defined as oligoprogressive on the basis that < two newly detected lesions had been detected during ARPI treatment. Conversely, patients who were included in the ARTO trial had upfront oligometastatic disease with < three non-visceral metastatic lesions, all undergoing SBRT. This could underline the fact that TRAP trial patients were affected by a higher burden of disease in comparison and that the ablative potential of SBRT may have been limited by the presence of untreated disease in this trial compared with ARTO.
Lastly, another limitation that may have affected the treatment provided within the TRAP trial consisted of a lower BED compared with the SBRT requirements for the ARTO trial patients (90Gy vs. > 100Gy with an alpha-to-beta ratio of 3).
These points of comparison are interesting when we consider the optimal maximisation strategy for SBRT addition to ARPi treatment. Thus, upfront treatment on all sites of oligometastatic disease early in the course of CRPC occurrence seems to be the best integration strategy to rely on, if compared with SBRT performed at a later stage of disease. An early multidisciplinary approach in CRPC patients appears to be the best treatment in order to ablate effectively all clones of the disease that may be progressing despite effective systemic hormonal treatment. Nonetheless, the TRAP trial results underline how, even in patients with adverse prognostic features (i.e. heavily pretreated, presence of multiple metastatic lesions with limited progressive sites of disease), SBRT may seem to be a viable and effective treatment. This could be of particular interest to those patients with limited systemic treatment options in the CRPC setting after ARPi and taxane chemotherapy, due to the absence of homologous recombinational repair pathway gene alterations.

Dr Giulio Francolini
Radiation Oncology Unit, Oncology Department, Azienda Ospedaliero Universitaria Careggi, Firenze, Italy.