ESTRO 2024 Congress
As local recurrences of prostate cancer (PCa) occur most often at the location of the macroscopic tumour(s) prior to treatment, a strategy for dose escalation is to deliver a simultaneously integrated boost to the intraprostatic tumour(s). It has already been shown in the focal lesion ablative microboost in PCa (FLAME) trial that focal boosting in 35 fractions can reduce the rate of biochemical recurrence in intermediate- and high-risk PCa (1). The trial of intensity-modulated fractionated radiotherapy vs. stereotactic body radiation therapy for PCa (PACE-B) showed that five-fraction prostate SBRT was not inferior to conventional radiotherapy for low- and intermediate-risk PCa (2).
The hypo-FLAME trial is a multicentre phase-II, single-arm trial that evaluated extreme hypofractionated doses of 35Gy applied in five weekly fractions to the whole prostate gland with an integrated boost of up to 50Gy to the MRI-defined tumour(s). The inclusion and exclusion criteria are listed in Table 1.
|
Inclusion criteria
|
Exclusion criteria
|
|
Intermediate or high-risk PCa (EAU)
|
Seminal vesicle invasion ≥ 5mm
|
|
Tumour nodule visible on multiparametric MRI
|
Serum total prostate-specific antigen > 30ng/ml
|
|
|
Prior pelvic radiotherapy/transurethral resection
|
|
|
N1
|
|
|
M1
|
|
|
Intl. prostate symptom score ≥15
|
EAU: European Association of Urology; N1: cancer has spread to nearby lymph nodes; M1: cancer has spread to distant parts of the body
The primary endpoint was acute toxicity and has already been reported (3). The secondary endpoints were the five-year biochemical disease-free survival (bDFS) rate and late toxicity as measured according to the common terminology criteria for adverse events (CTCAE) v4.0, and were the topic of this talk.
Between April 2016 and December 2018, 100 men were treated in four academic centres. Patients were mostly high-risk (75%). The choice to use androgen deprivation therapy (ADT) or not was left to the physician’s discretion, and was used in 62% of all patients. As reported before, priority was given to the protection of organs-at-risk (OARs), and this requirement resulted in a median dose to the MRI-defined tumour of 44.7Gy (interquartile range (IQR) 43.0-46.9), which was below the prescribed 50Gy. The maximal dose to the urethra (D0.035cc) was 39.3Gy (IQR 38.6-39.9). The five-year bDFS was 93%; only six out of 100 patients experienced recurrence. Three presented with distant recurrence only, two with regional recurrence, and one with both distant and local recurrence. Overall, ≥ Grade 3 genitourinary (GU) and gastrointestinal (GI) cumulative toxicity levels were 2% and 1%, respectively. These numbers were 12% and 4% for ≥ Grade 2 GU and GI toxicities (Figure 1).
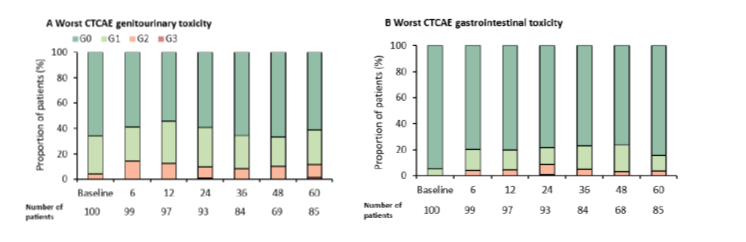
Figure 1. Late CTCAE toxicity by time-point (months). Grade distribution (%) of (A) GU and (B) GI toxicities
The authors concluded that five-fraction prostate SBRT with focal boost demonstrated excellent five-year efficacy outcomes in patients with mainly high-risk PCa while maintaining low rates of toxicity. The hypo-FLAME 3.0 trial will compare the five-year biochemical disease-free survival rates produced after the use of a moderately hypofractionated schedule with this five-fraction schedule. It will be very interesting to compare the results of the hypo-FLAME 3.0 trial with those of the PACE-C trial, as they are testing different dose fractionation schedules for the same intermediate- to high-risk population (Table 2).
|
|
Fractionation schedule
|
EQD2 equivalent (α/β = 1.5)
|
|
PACE C trial
|
60Gy/20 fractions
|
77.1Gy
|
|
36.25-40Gy/five fractions
|
90.6-108.6Gy
|
|
Hypo-FLAME 3.0 trial
|
62Gy/20 fractions
|
81.5Gy
|
|
35-50Gy/five fractions
|
85-164.3Gy
|
Table 2. Comparison of the fractionation schedules used in PACE C and hypo-FLAME 3.0 trials
References:
- Proffered Paper. Presented by Draulans C.: Focal boost SBRT in men with intermediate- and high-risk PCa: 5-year results of the hypo-FLAME trial
1. Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021;39(7):787-96.
2. van As N, Tree A, Patel J, Ostler P, Van Der Voet H, Loblaw DA, et al. 5-Year Outcomes from PACE B: An International Phase III Randomized Controlled Trial Comparing Stereotactic Body Radiotherapy (SBRT) vs. Conventionally Fractionated or Moderately Hypo Fractionated External Beam Radiotherapy for Localized Prostate Cancer. International Journal of Radiation Oncology, Biology, Physics. 2023;117(4):e2-e3.
3. Draulans C, van der Heide UA, Haustermans K, Pos FJ, van der Voort van Zyp J, De Boer H, et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother Oncol. 2020;147:92-8.
Dr Vérane Achard

Department of Radiation Oncology
Hôpital Fribourgeois, Fribourg, Switzerland
Department of Radiology and Medical Informatics
University of Geneva, Geneva, Switzerland