Register data support the long-term safety of the use of ultra-hypofractionated radiotherapy for localised prostate cancer previously seen in the phase 3 trial HYPO-RT-PC
ESTRO 2024 Congress report
The HYPO-RT-PC trial was the first randomised phase 3 trial to report five-year outcomes for the comparison between ultra-hypofractionated and conventionally fractionated radiotherapy in localised prostate cancer. Ultra-hypofractionation, that is, 42.7Gy in seven fractions delivered in three fractions/week over 2.5 weeks, had similar failure-free survival and long-term toxicity as conventional fractionation, 78.0Gy in 39 fractions with one fraction/day over eight weeks [1]. This Scandinavian trial enrolled 1200 patients who had intermediate- or high-risk localised prostate cancer in Sweden and Denmark between 2005 and 2015. Of these, 90% were Swedish residents.
Swedish registers hold diverse health data, for example on in-patient care, interventions, causes of death and drug prescriptions. By applying this opportunity to HYPO-RT-PC, indicators of morbidity from routine clinical practice could be compared between the arms to validate the trial’s toxicity results and counteract any loss to follow-up. Also, the results could be compared with men unexposed to prostate radiotherapy to estimate excess morbidity due to the radiotherapy.
In our study, presented at the conference [2], we set out to compare potentially radiotherapy-related genitourinary and gastrointestinal morbidity between the trial arms, and to estimate the excess morbidity due to prostate radiotherapy, using Swedish register data.
We included Swedish residents at trial enrolment and, to estimate excess morbidity, a matched group of men without medical histories of prostate cancer. Data were accessed from the Prostate Cancer data Base Sweden 5.0, which links the National Prostate Cancer Register to other population-based registers with a high coverage.
We defined potentially radiotherapy-related, medically significant genitourinary and gastrointestinal events using the Common Terminology Criteria for Adverse Events version 5, by mapping relevant descriptions of grades 3 and 4 toxicity to diagnosis and intervention codes. In-patient episodes of care, interventions and causes of death identified through the codes defined events (Fig. 1). Causes of death alone, minor genitourinary interventions (such as catheter use) and prolonged use of symptom-relieving medications were also compared.
At a median follow-up of 10.7 years, there were no statistically significant differences between the trial groups for any of the comparisons. Compared with the men without medical histories of prostate cancer, the excess genitourinary and gastrointestinal morbidity ranged between 3-10% at 10 years, but mortality from the conditions was low and similar. Therefore, our study adds further support regarding the safety of ultra-hypofractionated radiotherapy in localised prostate cancer.
In future research, our method could be used to investigate other important, related questions. As ultra-hypofractionation has been implemented in clinical practice in Sweden, it could be used to see whether the safety results are maintained when delivered outside the clinical trial setting. Also, it would be possible to explore whether the use of later improvements in radiotherapy techniques to treat localised prostate cancer, such as the routine use of volumetric modulated arc therapy, have translated into a reduction in morbidity.
.jpg.aspx?width=250&height=312)
Astrid E. Persson
Clinical oncology resident, PhD student
Department of Haematology, Oncology and Radiation Physics, Skåne University Hospital
Division of Oncology, Department of Clinical Sciences, Lund University
Lund, Sweden
astrid.persson@med.lu.se
www.linkedin.com/in/astrid-persson
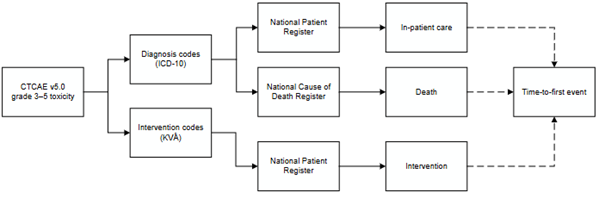
Fig. 1: Flow diagram shows the process for defining and identifying genitourinary and gastrointestinal events.
Abbreviations: CTCAE v5.0, Common Terminology Criteria for Adverse Events version 5; ICD-10, International Classification of Diseases, 10th revision; KVÅ, Classification of Care Measures.
References
[1] Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–95. https://10.1016/S0140-6736(19)31131-6.
[2] Persson AE, Kjellén E, Garmo H, Adrian G, Stattin P, Widmark A, et al. Register data on long-term morbidity after prostate ultra-hypofractionation in the HYPO-RT-PC trial. ESTRO 2024 Abstract Book [cited 2024 May 31]. Available from: https://user-swndwmf.cld.bz/ESTRO-2024-Abstract-Book/2295/.